Abstract
BACKGROUND AND PURPOSE: Transvenous embolization has emerged as a novel technique for treating selected brain AVMs with high reported occlusion rates. However, it requires anatomic and technical skills to be successful and to ensure patient safety. Therefore, training and testing are essential for preparing clinicians to perform these procedures. Our aim was to develop and test a novel, patient-specific brain AVM in vitro model for transvenous embolization by using 3D printing technology.
MATERIALS AND METHODS: We developed a brain AVM in vitro model based on real patient data by using stereolithography resin 3D printing. We created a closed pulsed circuit with flow passing from the arterial side to the venous side, and we tested the effect of mean arterial pressure on retrograde nidal filling with contrast injections. Transvenous embolization simulations were conducted for each of the 12 identical models divided into 2 groups (2×6). This involved the use of an ethylene-vinyl alcohol liquid embolic agent injected through microcatheters either without or with a coil in the vein (groups 1 and 2, respectively).
RESULTS: Retrograde contrast advance to nidus was directly related to lower mean arterial pressure. Transvenous embolization tests with a liquid embolic agent adequately reproduced the usual embolization plug and push technique. We found no differences between the 2 group conditions, and additional venous coil neither increased nidus penetration nor reduced injection time in the model (57.6 versus 61.2% nidus occlusion rate, respectively).
CONCLUSIONS: We were able to develop and test a functional in vitro brain AVM model for transvenous embolization by using 3D printing to emulate its conditions and characteristics. Better contrast penetration was achieved with less mean arterial pressure, and no embolization advantage was found by adding coil to the vein in this model.
ABBREVIATIONS:
- CAD
- computer-aided design
- EVOH
- ethylene-vinyl alcohol
- LEA
- liquid embolic agents
- MAP
- mean arterial pressure
- SLA
- stereolithography
- TVA
- transvenous approach
- TVE
- transvenous embolization
Endovascular embolization is a well-accepted treatment strategy for brain AVMs as either a standalone therapy or a part of multimodal management together with microsurgery or radiosurgery.1
The classical and more commonly used endovascular treatment is the transarterial approach of using microcatheters to inject liquid embolic agents (LEA) to fill the nidus and exclude it either partially or completely from the circulation.1⇓⇓-4
Since the early stages of brain AVM treatment, it has been a dogma to avoid occluding the vein until complete arterial or nidal control is achieved. An abrupt or unintended venous occlusion could result in an increase of intranidal pressure, which could potentially lead to secondary catastrophic bleeding.3,5 Despite these considerations, some theoretic conceptualizations, animal experiments, and clinical series showed that transvenous embolization (TVE) for brain AVM was feasible as a potential route for endovascular treatment.6,7 More recent series have shown TVE to be a transformative strategy for the curative endovascular treatment of selected cases, demonstrating much higher occlusion rates than those of the classic arterial route.8,9
The venous approach is technically demanding, and it requires thorough anatomic knowledge and highly developed endovascular skills. Endovascular training, material testing, and simulations for this access strategy have become an important need for achieving technical success and ensuring patient safety in recent years. Recently, Vollherbst et al,10 presented the first TVE in vivo model by using a swine rete mirabile. No other transvenous animal or in vitro model has been described thus far, though the latter have been widely used for other brain AVM endovascular treatment simulations.11⇓⇓⇓⇓⇓⇓-18
The purpose of this study was to present the development and testing of a novel in vitro brain AVM in vitro model for TVE by using stereolithography (SLA) 3D printing and real patient data.
MATERIALS AND METHODS
This research was approved by the local Institutional Review Board. An in vitro brain AVM model was designed based on real patient data. We selected a left temporal brain AVM that was under 3 cm in diameter with single superficial venous drainage (grade I, according to the Spetzler–Martin classification).19 A 3D rotational acquisition was performed by using an Icono biplane angiography system (Siemens Healthineers). The brain AVM vessel information was exported in DICOM format, and a manual segmentation process (region growing technique) was done by using 3D Slicer (https://www.slicer.org). The brain AVM structure with the nidus was defined and transformed to Standard Triangle Language. We created a container or “chip” where the brain AVM model was fitted via computer-aided design (CAD) software (Fusion 360, Autodesk and Rhinoceros 3D, Robert McNeel & Associates). For the brain AVM tubing designs, we used the nonuniform rational B-spline methodology, which allows the formation of an endoskeleton via a sweep of isogeometric hexahedral control meshes. These meshes are compatible with hemodynamic analyses and 3D rapid prototyping processes, which enables greater accuracy and efficiency in the design and simulation of the model. We simplified the model by removing the small structures that could not be adequately segmented, with the remaining smaller channels being between 0.5–1 mm. We added an external bypass channel that represented normal flow and luer lock connectors to the arterial input and venous output of the model.
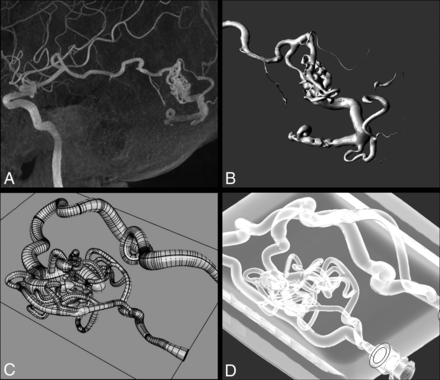
The final CAD model was exported for 3D printing. We used a commercial Form 3B printer (FormLabs) with transparent resin (Clear V4) to create the model by using stereolithography (Fig 1).
The process from the DICOM image to the model. A, MIP 3D reconstruction of the left temporal BAVM. Spetzler-Martin I. B, 3D image of the segmentation manual process by using the region growing technique with 3D Slicer Software. C, CAD representation of the BAVM model design after the main characteristics of the original disease. D, Final in vitro model inside the container, with transparent material and an external “normal” channel. BAVM indicates brain arteriovenous malformation.
We created a closed circuit setting of tubes and connectors with a pulsatile pump (FlowTek 125, United Biologics) that we used for all tests. The pump could modify the pulse and flow percentage, and the circulation was always from the arterial side to the venous side.
Transvenous Approach Injection Test
We aimed to determine the ideal pressure regimen of the system to allow counterflow to advance through the transvenous approach (TVA). The pulsatile pump was programmed with 60 cycles per minute, and 10 different flow percentages were tested: 70, 65, 60, 55, 50, 45, 40, 35, 30, and 25%. The circuit used NaCl 0.9% at 36°C. Pressure was measured using a transducer (TruWave, Edwards Lifesciences Services) in a 3-way connector at the entrance of the arterial part of the model and registered as the mean arterial pressure (MAP). We placed an Excelsior 1018 microcatheter (Stryker Neurovascular) at the venous collector, and iodinated contrast was manually injected (Visipaque 270, GE) under biplane fluoroscopy by using an Azurion 7 biplane (Philips Healthcare). We graded the contrast filling pattern by dividing the model into 4 different sections and assigning a score from 0–3 (with 0 representing no nidus filling and 3 representing the maximum retrograde contrast advance). The filling grade was compared with different MAP values by using the Pearson correlation test. The threshold for statistical significance was defined as P ≤ .05.
Transvenous Embolization Test
We aimed to recreate the conditions of TVE by using LEA in 2 different settings, and we evaluated the impact of venous coiling on the nidal occlusion rate, total embolization time, and number of stops after reflux. For this purpose, we used the brain AVM model connected to the pulsatile pump. Normal saline at 36°C was used to fill the system. All embolizations were done with a pulse of 60 cycles per minute and a flow rate of 30%, which resulted in a MAP of 21 mmHg and a systolic pressure of 74 mmHg. Under these settings, the flow of the model was 42 mL/min. Under biplane fluoroscopy, we performed retrograde LEA embolizations with an ethylene-vinyl alcohol (EVOH) copolymer (Squid 18, Balt). Two experienced (>10 years) interventional neuroradiologists performed the embolizations. We embolized 12 identical in vitro models that were separated into 2 groups:
Group 1. This group consisted of 6 models. A 1.5 F microcatheter (Apollo 1.5 30 mm, Medtronic) was navigated and positioned at the origin of the main vein over a 0.008″ micro-guidewire (Hybrid, Balt). The dead space of the microcatheter was flushed with dimethyl sulfoxide. LEA was injected through the microcatheter manually via the usual manner, taking 30-second pauses when reflux over the microcatheter was seen or when filling the arterial feeder up to the main arterial side. The procedure was stopped when the reflux was greater than the venous drainage or when no more nidus filling was seen after several injection and pause cycles.
Group 2. This group consisted of 6 models. A 1.5 F microcatheter was navigated and positioned in the same position as in group 1. A second microcatheter (Vasco 10+, Balt) was placed in a proximal position on the venous side, approximately 40 mm back from the first microcatheter tip. One bare platinum coil (Barricade 10×34 Frame complex coil; Balt) was deployed at this point using the “porcelain vein technique” as described by Mounayer’s group,9 in which EVOH is deposited in a centripetal and circumferential manner along the vessel wall during reflux to progressively reduce the inner diameter without immediately blocking the venous output. The LEA was then injected through the Apollo microcatheter using the same technique and injection/pause cycles as in group 1.
We measured the total injection time, number of pauses, and total injected LEA volume in both groups. We also determined the occlusion percentage of the nidus by using a 3D rotational acquisition without contrast at the end of the embolization procedure. The LEA of the model was segmented using the 3D Section software package, and the total filling volume was calculated using the PreForm software package (FormLabs). The percentage of nidal occlusion was calculated by using the total nidal volume from the CAD model minus the calculated nidal LEA volume from the 3D rotational images.
We used the Mann-Whitney U test to evaluate the differences between the 2 groups. The threshold for statistical significance was defined as P ≤ .05.
RESULTS
TVA Injection Test
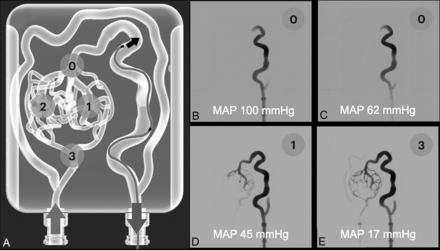
We found a statistically significant correlation between the MAP and contrast media retrograde filling through the brain AVM model (P = .001) (Fig 2). With lower MAP values, greater contrast nidal penetration and higher filling scores were found.
Contrast media retrograde test. Injections were done by using an Echelon 10 microcatheter and iodinated contrast. A, We tested 10 different input pressures from 10 different flow percentages, and we graded the retrograde advance from 0–3 as follows: 0 = no significant advance; 1 = 1 venous limb complete filling; 2 = 2 venous limbs complete filling; 3 = complete venous filling plus proximal arterial filling. B–E, Retrograde contrast injections with different MAP values. An increase in nidal contrast penetration was seen with lower MAP values, with a statistically significant correlation (P = .001).
Transvenous Embolization Test
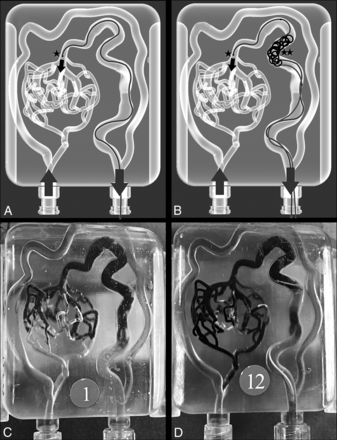
LEA was injected retrogradely to the model by using EVOH in 2 groups with the described techniques (Fig 3). No differences were found between group 1 (no coil on the venous side) and group 2 (bare platinum coil on venous collector) in any of the different measured variables (Table).
Transvenous embolization tests. TVE techniques were performed on 12 identical AVM models, divided into 2 groups (2 × 6). A, Group 1, where retrograde embolization was done by using an Apollo 1.5 30 mm microcatheter (★). B, Group 2, with the same position of the Apollo 1.5 30 mm (★) and a venous coil deployed with a Vasco+ microcatheter in a proximal vein position (★★). C, Real photograph after the EVOH embolization of a group 1 case. D, Real photograph after the EVOH embolization of a group 2 case.
Transvenous embolization resultsa
Embolizations were done by using the usual plug and push technique. Transparent resin material allowed a direct and easy visualization of the embolization process. Digital subtraction angiography controls performed at the end of the embolization session showed no nidal contrast filling in any treated model.
DISCUSSION
Transvenous embolization has been described and increasingly used in recent years, and it has been demonstrated to be an outstanding approach by which to achieve a brain AVM cure in highly selected patients. Large series have showed high nidal occlusion rates and similar safety profiles compared with the usual endovascular trans-arterial approaches.9,20 This evolving technique requires the proper training and teaching of physicians, development of specific devices, and testing of new materials. Various models have been used for decades for these purposes, with the swine rete mirabile being the model of choice for endovascular training and testing.21⇓-23 In vitro models have been less explored to simulate brain AVM embolizations due to several limitations, including difficulties in defining the real nidal anatomy by using the currently available imaging modalities, complicated segmentation processes, and the complexity of building small hollow channels. Several in vitro models have been described since late 1970s for endovascular use by using different materials and structures that could resemble a brain AVM or nidus, from straight tubes to honeycomb-like structures as well as passing through tubes filled with beads, scouring pads, sponges, blood filters, or springs.13⇓⇓⇓-17,24,25 A search for more realistic features has been done in the last years. Kaneko et al12 described an in vitro brain AVM model with realistic features and hollow channels by using 3D printing techniques in combination with solid printing and silicon coverage ready for LEA embolizations after the dissolution of inner solid compounds. Our group recently described a new, different technology process by using SLA 3D printing and millifluidic techniques that allow us to create an in vitro brain AVM model with small hollow channels that can be used for training and endovascular simulations.18 These in vitro models have been used to replicate arterial embolization treatments, but no TVE approaches have been performed. As previously stated, the only TVE model described thus far in the literature has been a swine rete mirabile in vivo model.10 In vivo models are of great importance and utility for endovascular training, but the management, care facilities, transportation, costs, and ethical aspects regarding the use of animals for training, testing, or education present great barriers for their massive use.
In this study, we were able to present a novel in vitro brain AVM model for TVE. We were able to use the AVM data of a real patient and translate it to a 3D-printed SLA container. This process is an evolution of our earlier described SLA model, in which we created a simulated nidus anatomy.18 This is a first step, and we are aware that many challenges remain in adequately reproducing true nidal structures, but the current model opens many possibilities for training, rehearsal, and material testing, as has previously been done for brain aneurysms.26⇓⇓-29 Our new in vitro model has smaller hollow channels and more realistic structures and vessels. We expect more advances in the near future as acquisition imaging techniques and 3D printing technologies continue to improve and will eventually allow for the creation of smaller hollow or tubular structures.
Counterflow contrast injection proved to be in direct relation to MAP. Our model allowed us to define different pressure settings, and nidus retrograde filling was larger as systemic pressure was lower, with a statistically significant correlation. This concept was first described by Massoud et al6 with their transvenous retrograde nidus sclerotherapy under controlled hypotension technique of injecting contrast in a retrograde manner in a swine rete mirabile model. Contrast retrograde penetration was greater with lower MAP pressure values.6 The same concept has been applied in real TVE cases to create local or systemic hypotension for fast and complete nidal penetration, such as with proximal arterial balloon inflation, cardiac rapid ventricular pacing, and systemic induced hypotension.9,20,30
The TVE in our model behaved similar to that in real cases. As expected, the navigation of the microcatheters was realistic and plausible with some extra friction in the in vitro model.26 The injection of LEA began with some reflux to the vein, after which it began to advance through the nidus in the plug and push fashion. A plug rapidly was created, and fast and initial filling of the nidus was possible. Secondary reflux began and was controlled as much as possible with 30-second stops until our limit reflux was reached. We expected to have different occlusion results between our 2 experimental groups, with faster and greater nidal penetration in the second group, in which we used coil in the venous side. Nevertheless, we found no statistically significant difference between the two groups. We hypothesize that the absence of clot formation within the coil (because of the use of NaCl 0.9% to replace blood) and the limited number of coils used (1 long coil) may explain these results, as these are factors that contribute to stronger and better vein flow control. Our aim was to standardize the procedure and test the porcelain vein technique, so no other materials were used as glue, as described for the venous pressure cooker.20 The nidal occlusion percentage was near 60%, though we expected to observe greater penetration and filling. However, we did see complete angiographic exclusion at the end of the procedure in all cases.
Our model presents other limitations. It is a solid in vitro model, so there were no elastic features as real vessels, and the model could not simulate procedure-related rupture as with an in vivo brain AVM. Friction inside the model felt higher than that of human vessels, but microcatheter navigation was still adequately possible. Although we created a model based on a real brain AVM, there are still anatomic limitations of the reproducibility, and we could not replicate some smaller vessels because of actual image resolution, segmentation, and hollow vessel printing. The number of tested models was limited, and we did not test other flow control techniques at the venous side that could have contributed toward better nidal occlusion penetration, nor did we control proximal arterial flow with balloons.
However, despite some intrinsic limitations, we believe that our model presents several advantages as the model size, reproducibility, and easy transportation facilitate setup in any place or angiographic suite. The model is transparent, and though we did not actively test this feature, embolization is feasible and could be controlled without the use of X-rays. The procedure could be well-tested under direct vision or enhanced using a camera or video magnification.
Finally, we have successfully created a functional in vitro brain AVM model for TVE by using 3D printing to simulate its conditions and characteristics. This model can be used for training, educational purposes, and testing novel techniques and materials.
Acknowledgments
We would like to thank Balt (Montmorency, France) for donating some of the materials that were used during the embolization tests as Squid 18 and Barricade coils.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received October 17, 2023.
- Accepted after revision January 11, 2024.
- © 2024 by American Journal of Neuroradiology