SUMMARY:
The clinical standard of care in the diagnosis of neurodegenerative diseases relies on [18F] FDG-PET/CT or PET MR imaging. Limitations of FDG-PET include cost, the need for IV access, radiation exposure, and availability. Arterial spin-labeling MR imaging has been shown in research settings to be useful as a proxy for FDG-PET in differentiating Alzheimer disease from frontotemporal dementia. However, it is not yet widely used in clinical practice, except in cerebrovascular disease. Here, we present 7 patients, imaged with our routine clinical protocol with diverse presentations of Alzheimer disease and other neurodegenerative diseases, in whom arterial spin-labeling–derived reduced CBF correlated with hypometabolism or amyloid/tau deposition on PET. Our case series illustrates the clinical diagnostic utility of arterial spin-labeling MR imaging as a fast, accessible, and noncontrast screening tool for neurodegenerative disease. Arterial spin-labeling MR imaging can guide patient selection for subsequent PET or fluid biomarker work-up, as well as for possible therapy with antiamyloid monoclonal antibodies.
ABBREVIATIONS:
- AD
- Alzheimer disease
- ADLs
- activities of daily living
- ASL
- arterial spin-labeling
- CI
- cognitive impairment
- COVID-19
- coronavirus 2019
- lvPPA
- logopenic variant progressive aphasia
- MCI
- mild cognitive impairment
- MoCA
- Montreal Cognitive Assessment
- PCA
- posterior cortical atrophy
Neurodegenerative disease, of which Alzheimer disease (AD) is the most common, is the leading cause of dementia and manifests as progressive cognitive impairment (CI) along with a variety of other neurologic symptoms.1 Standard-of-care MR imaging can exclude potentially treatable, structural causes of CI and can identify atrophy but lacks sensitivity and specificity. In the era of antiamyloid monoclonal antibody therapy for AD, identifying reliable and easily accessible diagnostic tools for neurodegenerative disease is of paramount importance.
FDG-PET is routinely used clinically to differentiate AD from other neurodegenerative diseases.1 Recently, the Centers for Medicare and Medicaid Services decreased the restrictions on the coverage of amyloid PET, providing more access to patients seeking antiamyloid therapies.2 However, both FDG and amyloid PET scans are costly, requiring IV access for radiotracer administration, radiation exposure, and proximity to a PET facility.
Arterial spin-labeling MR imaging (ASL-MR) is a noncontrast MR imaging technique that uses endogenous water in arterial blood for a qualitative and quantitative assessment of CBF.3 Its utility as a surrogate measure of neuronal activity and metabolism on FDG-PET has been described in AD research settings, particularly at the group level;4 however, it has thus far not been demonstrated in clinical practice.
We present 7 cases of neurodegenerative disease (6 with forms of AD) in which a clinical MR imaging protocol to evaluate cognitive impairment that included a 4.5-minute ASL MR image was effective at predicting findings on subsequent PET, even when structural imaging was not revealing. Full informed consent was obtained from all patients. We propose that ASL-MR could be a cost-effective AD screening and longitudinal follow-up tool.
CASE SERIES
Case 1: Early-Onset AD with Presenilin Mutation
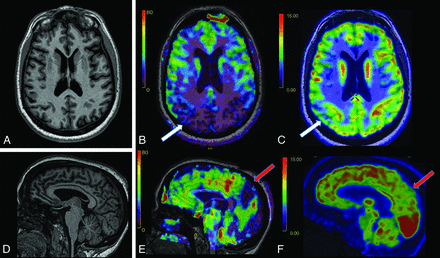
A 57-year-old postmenopausal woman with a history of thyroid cancer and hyperlipidemia presented with slowly progressive short-term memory loss for 10–15 years, as well as anxiety and fatigue for 1–2 years. She was independent in activities of daily living (ADLs). Her father and 3 paternal siblings had onset of dementia at ages 50–60 years. Two siblings were cognitively healthy. Neurologic assessment showed mild cognitive impairment (MCI), a Montreal Cognitive Assessment (MoCA) score of 16/30, and mild parietal drift. Serum labs were noncontributory. Structural MR imaging showed nonspecific mild volume loss. ASL-MR showed decreased bilateral temporoparietal CBF, including in the precuneus (Fig 1 and Online Supplemental Data); FDG-PET corresponded to these findings (Fig 1 and Online Supplemental Data). Amyloid PET revealed diffuse cortical amyloid deposition (Online Supplemental Data). Genetic testing revealed a heterozygous PSEN1 mutation (c.617G>C hereditary autosomal dominant AD). The patient has been prescribed lecanemab.
Early-onset AD with presenilin mutation. A, Axial 3D T1 MPRAGE image shows mild generalized volume loss. Axial CBF image (B) and axial FDG-PET image (C) show more pronounced decreased CBF than FDG avidity in the bilateral parietal lobes, particularly on the right (white arrow), and temporal lobes (not pictured), suggestive of AD. D, Sagittal 3D T1 MPRAGE image shows nonspecific volume loss. Sagittal CBF image (E) and sagittal FDG-PET image (F) show decreased CBF and FDG avidity in the precuneus (red arrow).
Case 2: Early-Onset AD and Long COVID Brain Fog
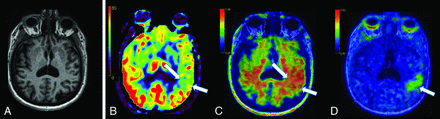
A 53-year-old woman with pre-existing hypertension and seronegative presumed rheumatologic disease presented with “brain fog,” insomnia, orthostasis, and headaches after mild coronavirus 2019 (COVID-19) infection. Following a COVID-19 vaccination, she developed Bell palsy and peripheral herpes simplex virus reactivation. She had previously worked full-time in a cognitively demanding position. Maternal family history was positive for AD onset around the age of 60. She had a MoCA score of 29/30, a Mini-Mental State Examination score of 30/30, and facial nerve findings of Bell palsy. The initial clinical diagnosis was post-COVID MCI, with worsened rheumatologic disease. However, subsequent ASL-MR demonstrated asymmetrically decreased left temporoparietal CBF (Fig 2 and Online Supplemental Data), with confirmed β-amyloid deposition on [18F] florbetaben PET and tau deposition on [18F] MK-6240 PET, consistent with a diagnosis of AD (Fig 2 and Online Supplemental Data). There was also evidence of β-amyloid deposition in the parietal lobes bilaterally, but no other areas of tau deposition (Online Supplemental Data). The patient was prescribed lecanemab.
Early-onset AD and post-COVID brain fog. A, Axial 3D T1 MPRAGE image shows mild volume loss, more prominent in the left hemisphere. B, Axial CBF image shows asymmetrically decreased CBF in the left temporoparietal junction (white arrows). C, Axial [18F] florbetaben PET image shows asymmetric cortical deposition of β-amyloid (white arrows). D. Axial [18F]-MK6240 tau PET shows focal cortical deposition of tau (white arrows), corresponding to the area of lowest CBF noted in B and β-amyloid deposition in C.
Case 3: Posterior Cortical Atrophy—the “Visual Variant” of AD
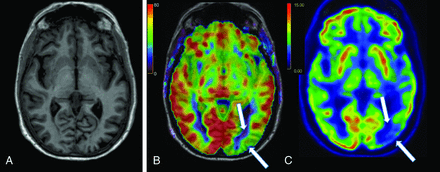
A 74-year-old woman with pre-existing hyperlipidemia, depression/anxiety, and insomnia presented with progressive forgetfulness for 2 years, as well as disorientation, worsened anxiety, and orthostasis for 4–7 years. She continued to work full-time. Examination revealed a MoCA score of 28/30, abnormal findings on bilateral Humphrey visual field testing, a left parietal drift, proprioceptive deficit and paratonia, and postural instability. MR imaging showed subtle left occipital lobe atrophy, with associated decreased CBF (Fig 3 and Online Supplemental Data). FDG-PET demonstrated corresponding decreased avidity, compatible with posterior cortical atrophy (PCA) (Fig 3 and Online Supplemental Data). The patient is considering lecanemab.
PCA, the “visual variant” of AD. A, Axial 3D T1 MPRAGE image demonstrates mild left occipital lobe atrophy. B, Axial CBF image shows asymmetrically decreased CBF in the left occipital lobe (white arrows), with corresponding decreased avidity on the axial FDG-PET image (C).
Case 4: Early-Onset PCA
A 55-year-old man with a history of hypertension and prior alcoholism, complicated by an episode of withdrawal seizures, presented with 1 year of “long COVID brain fog.” The onset of symptoms was subacute after mild COVID-19 infection. Deficits included worsening dysgraphia, spatial disorientation, visual scanning deficit, simultagnosia, finger agnosia, apraxia, short-term memory lapses, and depression/anxiety, all causing disability; ADLs were preserved. His MoCA score was 7/30, complicated by severe test anxiety. CSF was borderline for AD (amyloid β 42 = 385 pg/mL, phosphorylated tau = 62.1 pg/mL, amyloid-to-tau index = 0.61). MR imaging showed moderate generalized atrophy, with decreased CBF corresponding to areas of cortical tau deposition on subsequent tau PET (Online Supplemental Data). Involvement of the left occipital lobe and visual symptoms were consistent with the clinical diagnosis of PCA, the visual variant of AD.
Case 5: Early-Onset Logopenic Variant Primary Progressive Aphasia, the “Language” Variant of AD
A 54-year-old woman presented with progressive short-term memory loss, word-finding difficulty, difficulty navigating streets, and trouble separating languages for the past several years. She remained working as a home nurse attendant. She had prediabetes, hyperlipidemia, and anxiety on sertraline. Her mother and maternal aunt had dementia in their 60s. She had occasional orientation deficits but full ADLs, a MoCA score of 15/30, trouble understanding complex commands, bradyphrenia, mild paratonia, and mild optic ataxia. Findings of a genetic dementia panel were negative. ASL-MR demonstrated decreased bilateral parietal and left temporal CBF (Online Supplemental Data), correlating to areas of decreased FDG-PET avidity, compatible with logopenic variant progressive aphasia (lvPPA) (Online Supplemental Data). This patient is a lecanemab candidate and is awaiting insurance coverage for an amyloid PET scan.
Case 6: lvPPA
A 76-year-old man presented with a progressive expressive language deficit, short-term memory loss, visual scanning difficulty, executive dysfunction, and difficulty with higher-level thinking for 4 years. He had hypertension, hyperlipidemia, and 2 maternal aunts with dementia in their 80s. He required reminders for some ADLs but could still follow current events. While his MoCA score was 4/30, he expressed complex concepts despite bradyphrenia, read with some hesitation, wrote, and repeated back risks of therapies. He had a mild postural tremor and ataxic gait. ASL-MR demonstrated decreased bilateral parietal, left-frontal and left-temporal CBF (Online Supplemental Data), more pronounced in extent than the degree of hypometabolism on FDG-PET (Online Supplemental Data). The asymmetric pattern and language deficit were compatible with lvPPA. Amyloid PET revealed diffuse cortical amyloid deposition (Online Supplemental Data).
Case 7: Progressive Supranuclear Palsy
A 59-year-old woman presented with progressive speech difficulty, short-term memory loss, apraxia, anxiety, and falls, progressing for 3 years and now needing reminders for ADLs. Her MoCA score was 15/30; she had prominent perseveration, dysarthria, phonemic paraphasia, terseness, and Parkinsonism with limited upgaze and postural instability. CSF protein and myelic basic protein were slightly elevated. MR imaging showed midbrain atrophy, suggestive of the “hummingbird” sign of progressive supranuclear palsy. This diagnosis was supported by decreased CBF and FDG avidity in the frontal lobes and [123I] loflupane SPECT (DaTscan; GE Healthcare) findings indicating reduced radiotracer activity in the left caudate and left putamina greater than the right (Online Supplemental Data).
DISCUSSION
Our case series, which includes early and late onset of amnestic, visual, and language variants of AD and atypical parkinsonism, illustrates the clinical utility of ASL-MR in the management of individual patients with MCI or mild dementia. Most of the patients presented here had only mild, nonspecific volume loss on the volumetric T1-weighted sequence. However, ASL-MR was abnormal in all patients, with specific areas of decreased CBF corresponding to hypometabolism (cases 1, 3, 5, 6, and 7) or amyloid and tau deposition (cases 1, 2, 4, and 6) on PET. These findings lend strong support for an add-on, 4.5-minute, clinical noncontrast ASL-MR image to increase the sensitivity and utility of a routine clinical standard-of-care MR imaging for cognitive impairment.
The utility of ASL-MR as a proxy for FDG-PET in neurodegenerative disease has previously been described primarily in research settings. The concept that decreased CBF should approximate hypometabolism on FDG-PET arose from the observation that perfusion and metabolism in the brain are tightly coupled.5 Indeed, studies in patients with AD that used both ASL-MR and FDG-PET found a high degree of overlap between the abnormalities found on the 2 modalities.4,6 Additionally, studies in small AD cohorts have reported similar diagnostic accuracies between ASL-MR and FDG-PET.7,8 However, ASL-MR is still not yet widely used in the clinical realm for this indication. One reason may be that MR imaging in neurodegenerative disease has traditionally been used largely to exclude treatable causes of dementia, because the sensitivity and specificity of structural MR imaging alone for diagnosing AD is modest and no previous disease-modifying AD treatments existed. Furthermore, ASL-MR relies on arterial blood water as an endogenous tracer, which results in a low SNR, initially limiting radiologists’ confidence. However, image quality has improved substantially, supporting the clinical use, as shown in our case series.
An added novelty of our case series compared with the existing literature is that we included atypical variants of neurodegenerative disease. Thus, we demonstrate the diagnostic utility of different patterns of decreased CBF and their correlation with subsequent PET findings to illustrate the clinical utility of ASL-MR beyond the typical Alzheimer pattern. Our findings are concordant with the previously demonstrated advantage of ASL-MR in its ability to identify hypoperfusion before substantial volume loss, often exhibiting greater degrees of CBF reductions than grey matter tissue loss in affected regions (Online Supplemental Data).9 In some cases, CBF changes were more pronounced than their subtle FDG-PET hypometabolic correlates; this finding also suggests the high sensitivity of ASL-MR. Furthermore, we provide evidence for spatial overlap of CBF abnormalities and PET amyloid and tau deposition, suggesting that CBF can reflect underlying disease pathology.
The utility of FDG-PET in the diagnosis of atypical parkinsonism syndromes has been described, but ASL-MR has been described less.10 One study described CBF differences in Parkinson disease versus Parkinson-Plus syndrome but did not differentiate among the Parkinson-Plus syndromes.11 Case 7 in our series exemplifies the utility of ASL-MR in identifying frontal hypoperfusion, which corresponds to FDG-PET findings of frontal hypometabolism in progressive supranuclear palsy. While the hummingbird sign on structural MR imaging is specific for a diagnosis of progressive supranuclear palsy, frontal hypometabolism seen on FDG-PET has been shown to correlate with disease duration and cognition, adding prognostic value beyond structural MR imaging.12
In summary, our case series is a small representation of our growing patient cohort that has received a standardized, clinical MR imaging protocol including ASL-MR and has demonstrated a spectrum of clinical severity of underlying Alzheimer disease and other neurodegenerative pathologies, from subjective impairment to MCI to dementia. We have found that ASL-MR is a highly sensitive screening tool for these pathologies. We have used CBF abnormalities to guide us toward further work-up, either via FDG-PET, amyloid/tau PET, and/or CSF sampling. In our case series, there was limited cerebrovascular burden (Online Supplementary Data), but future work can consider the impact of comorbid severe vascular disease on CBF.13 Additionally, CBF is only a surrogate measure of neuronal activity based on neurovascular coupling,14 so future work will evaluate instances of discordance between CBF and FDG and underlying pathophysiologic mechanisms for this discordance. Nevertheless, incorporation of this cost-effective technology into cognitive screening in the primary care or neurology clinical setting could translate into a profound population health impact, especially at a time when disease-modifying treatment is available, including the antiamyloid monoclonal antibody therapies, such as lecanemab.15,16 These interventions are possibly most effective when initiated early, even presymptomatically, strengthening our case for the need to identify effective screening modalities.16
CONCLUSIONS
Our case series is concordant with prior knowledge that functional changes typically precede structural changes in patients with neurodegenerative disease. In each case of neurodegenerative disease presented, ASL-MR was an appropriate proxy for FDG-PET findings and, in some cases, amyloid and tau deposition. These patients are a small sample of our larger cohort of patients with CBF correlates to neurodegenerative disease with a wide spectrum of presentations. With standardization in clinical practice and technologic advancements in ASL-MR, CBF has become a useful screening tool. Further investigations are necessary to support the widespread systematic deployment of this efficient and cost-effective cognitive screening tool in the race to identify, treat, and perhaps even monitor longitudinal change and treatment response in neurodegenerative disease from its earliest stages.
Footnotes
G.C. Chiang and A.S. Nordvig contributed equally to this article.
This work was supported by National Institute of Neurological Disorders and Stroke NeuroNEXT Fellowship Award 5U24NS107168, National Institutes of Health/National Institute on Aging R01 AG068398, National Institutes of Health/National Institute on Aging R01 AG080011.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 8, 2023.
- Accepted after revision January 3, 2024.
- © 2024 by American Journal of Neuroradiology