SUMMARY:
Peripheral trigeminal neuropathies are assessed by MR neurography for presurgical mapping. In this clinical report, we aimed to understand the utility of MR neurography following nerve-repair procedures. We hypothesized that postoperative MR neurography assists in determining nerve integrity, and worsening MR neurography findings will corroborate poor patient outcomes. Ten patients with peripheral trigeminal neuropathy were retrospectively identified after nerve-repair procedures, with postsurgical MR neurography performed from July 2015 to September 2023. Postsurgical MR neurography findings were graded as per postintervention category and subcategories of the Neuropathy Score Reporting and Data System (NS-RADS). Descriptive statistics of demographics, inciting injury, injury severity, NS-RADS scoring, and clinical outcomes were obtained. There were 6 women and 4 men (age range, 25–73 years). Most injuries resulted from third molar removals (8/10), with an average time from the inciting event to nerve-repair surgery of 6.1 (SD, 4.6) months. In Neuropathy Score Reporting and Data System-Injury (NS-RADS I), NS-RADS I-4 injuries (neuroma in continuity) were found in 8/10 patients, and NS-RADS I-5 injuries were found in the remaining patients, all confirmed at surgery. Surgeries performed included microdissection with neurolysis, neuroma excision, and nerve allograft with Axoguard protection. Three patients with expected postsurgical MR neurography findings experienced either partial improvement or complete symptom resolution, while among 7 patient with persistent or recurrent neuropathy on postsurgical MR neurography, one demonstrated partial improvement of sensation, pain, and taste and one experienced only pain improvement; the remaining 5 patients demonstrated no improvement. Postsurgical MR neurography consistently coincided with clinical outcomes related to pain, sensation, and lip biting and speech challenges. Lip biting and speech challenges were most amenable to recovery, even with evidence of persistent nerve pathology on postsurgical MR neurography.
ABBREVIATIONS:
- MRN
- MR neurography
- NS-RADS I
- Neuropathy Score Reporting and Data System Injury
- NS-RADS PI
- Neuropathy Score Reporting and Data System Postintervention
- NST
- neurosensory testing
- PI
- postintervention
- PTN
- peripheral trigeminal neuropathy
The trigeminal nerve is the largest of the cranial nerves that provide sensory innervation to the face in addition to motor innervation to the muscles of mastication.1 It arises from the anterolateral aspect of the midpons with a dominant sensory and a smaller motor branch before dividing at the trigeminal ganglion into the 3 branches: ophthalmic, maxillary, and mandibular. These subdivisions further branch into smaller nerves that provide innervation to their respective maxillofacial distributions. For example, the inferior alveolar nerve innervates the lower jaw, and the lingual nerve innervates the tongue.2
Peripheral trigeminal neuropathies (PTNs) result in loss of sensation and/or the development of neuropathic pain, and these can be caused by both traumatic (third molar removal, oral implants) and nontraumatic (neoplastic, atypical facial pain) etiologies. The most common cause is third molar extractions with up to 10 million being performed each year, accounting for 60% of nerve injuries around the jaw. Among the trigeminal nerve branches, the lingual nerve, including the special sensory branch of the chorda tympani (cranial nerve VII), and the inferior alveolar nerve divisions of the mandibular nerve are most affected. Although the rate of permanent paresthesia in third molar extraction is relatively low at 0.33%, with ∼3.5 million lower third molar extractions performed annually in the United States, the incidence of permanent paresthesia can be 11,500–35,000 persons per year.3⇓⇓⇓⇓-8 The relatively high incidence rate of these injuries, in association with their notable pain and loss of function, leads to substantial negative effects on the quality of life.9
The current reference standard for the diagnosis and monitoring of PTNs is centered around neurosensory testing (NST) in combination with the clinical findings. With NST, 3 nerve-function domains are evaluated, including the spatiotemporal sensory domain, monofilament contact detection, and pain tolerance/thresholds.8 NST results are used to determine the severity of nerve injury based on the Sunderland classification criteria, which categorize nerve injuries in classes I–V, depending on the damage to the components of the nerve structure (myelin loss, axonal damage, and damage to the endoneurium, perineurium, and epineurium in that order).10,11 While NST has been useful in the diagnosis and monitoring of PTNs, various limitations exist, such as operator dependence, patient subjectivity, minimal anatomic specificity, and the inability to determine patient recovery after nerve surgery.8,12 MR neurography (MRN), an imaging technique dedicated to the evaluation of peripheral nerves, provides a 3D map of the neural anatomy like MRA. It has been validated for determining the Sunderland classification of nerve injuries preoperatively, with moderate-to-good correlation with NST and surgical findings for both the inferior alveolar nerve and lingual nerve injuries.13⇓-15 MRN results are often achieved earlier in the posttraumatic setting than NST results, aiding in the prompt diagnosis of more severe injuries and improved patient outcomes.16
Current research on the utility of MRN in the management of nerve injuries including PTN has largely focused on its uses in the preoperative diagnostic setting. NST is extremely limited and not useful in the postoperative setting due to variable pain and sensory responses. In this clinical report, we aimed to determine the utility of MRN in monitoring of structural changes following nerve-repair procedures. We hypothesized that postoperative MRN can assist in determining nerve integrity, and worsening MRN findings will corroborate poor patient outcomes.
CASE SERIES
This report was retrospective and performed under institutional review board approval. Informed consent was waived, and all Health Insurance Portability and Accountability Act of 1996 regulations were followed.
MATERIALS AND METHODS
Patient Population and Study Sample
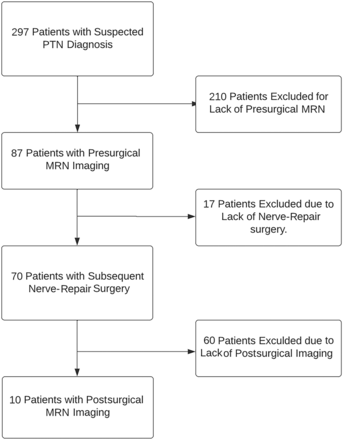
Two hundred ninety-seven consecutively sampled adult patients of both sexes with suspected PTNs were retrospectively queried from the institution database during an 8-year period (July 2015 to September 2023). All these patients were referred from the institutional Oral and Maxillofacial Surgery Clinic. They were evaluated by an experienced maxillofacial surgeon (J.R.Z,) and underwent trigeminal NST with subsequent trigeminal MRN imaging. From this patient population with PTN, 210 patients were excluded because they did not undergo presurgical MRN, and an additional 17 were excluded because they did not have a corresponding nerve-repair procedure. Of the 70 remaining patients, 60 patients were excluded due to lack of postsurgical MRN. The 10 remaining patients had postoperative MRN, all interpreted by a single fellowship-trained radiologist (A.C.) with >15 years of experience in musculoskeletal radiology and extensive expertise in MRN.
Patient charts were evaluated for the inciting event and presurgical clinical and NST findings with their corresponding Sunderland classification (classes I–V). The clinical findings captured in this review included pain, sensory abnormalities, taste changes, and associated functional changes (ie, lip biting and speech challenges). All patients underwent presurgical MRN at the same institution using a uniform protocol and a 32-channel head coil, with interpretation performed blinded to clinical NST findings and before surgical intervention (see Table 1 for MRN protocol details). The scans were obtained on 3T and 1.5T scanners (Achieva and Ingenia; Philips Healthcare), and MIP reconstructions were performed using a slab thickness of 10 mm in the oblique coronal and sagittal planes along the respective nerve course for its long-axis depiction. A review of presurgical MRN imaging identified the severity of nerve injury by the Neuropathy Score Reporting and Data System Injury (NS-RADS I 1–5) criteria.17 Surgical notes were then reviewed for procedures performed, surgical findings, time elapsed from inciting event to surgery date, and injury severity according to the Sunderland classification.
MRN protocol on 3T scanner (Ingenia, Achieva)
When we reviewed Sunderland classifications, findings of patients not specifically stratified into 1 class (for example, class III/IV or class IV/V) at the time of the initial presentation were recorded as inconclusive, and patients who were not clinically tested for various reasons (severe pain or inability to open the mouth in acute injury) were considered unclassified for the purpose of this clinical report. Inconclusive MRN classifications were re-evaluated and assigned a specific nerve injury classification on re-evaluation by an experienced radiologist.
A corresponding review of postoperative MRN findings for each patient was performed and compared with the postoperative clinical findings. Postsurgical MRN findings were categorized by the Neuropathy Score Reporting and Data System Postintervention (NS-RADS PI) score, with NS-RADS PI-1 showing expected postsurgical findings, NS-RADS PI-2 demonstrating possible persistent neuropathy, and NS-RADS PI-3 demonstrating definitive recurrent or persistent neuropathy, including the formation of new neuromas, in accordance with the MR imaging reporting guidelines of peripheral neuropathy.17 Postsurgical MRN was interpreted by the same expert radiologist blinded to postsurgical clinical information and outcomes.
Clinical and Surgical Classifications
All NSTs and surgical procedures were performed by the same experienced maxillofacial surgeon using the same allograft technique for nerve reconstructions, apart from neurolysis. Postsurgical clinical findings evaluated were identical to their presurgical counterparts and were categorized on the basis of their status (no change, partial improvement, complete resolution).
Statistical Analysis
Descriptive statistics were used for demographic information (age, sex, and so forth), inciting injury, injury severity, and comparing postsurgical neuropathy recovery with findings on postsurgical MRN using Neuropathy Score Reporting and Data System scoring and clinical outcomes. We only obtained descriptive statistics due to having a small sample with 3 different subcategories of postintervention (PI) states. Concordance was defined as resolution or partial improvement of symptoms in an MRN-improved state (PI-1) and persistence or worsening of symptoms in PI-2/3 states.
RESULTS
Patient Population and Study Sample
Of the continuously sampled 297 patients with PTN initially reviewed, 227 were excluded due to lack of surgical intervention, and 60 of the remaining 70 patients were excluded because they had no postsurgical MRN (See Fig 1 for details of exclusion and inclusion criteria of the patient population). Among the final sample of 10 patients reviewed, 6/10 were women with mean ages of 44.8 (SD, 16.8) years with an age range of 25–73 years for all patients. Iatrogenic trauma was the inciting event for all patients, with third molar removal being the most common cause, occurring in 8/10 patients. The remaining 2 patients underwent multiple surgeries: one having various procedures and revisions involving the temporomandibular joint and the other having prior nerve-repair procedures related to the inferior alveolar nerve and mental nerve. The lingual nerve was injured in 8/10 patients, while injury to the mental nerve and infraorbital nerve was present in 1 patient each. The presurgical neuropathy symptoms of hypoesthesia/anesthesia and burning pain were each found in 7 patients, while hypogeusia/ageusia and dysgeusia were found in 6 and 4 patients, respectively. Presurgical neuropathy functional symptoms of lip biting were found in 7 patients. Speech difficulties were noted in 3 patients, and chewing challenges were seen in 2 patients.
Patient population in this study.
Clinical and Surgical Classifications
Of the patients with presurgical NST, 8/10 patients were classified by the Sunderland classification criteria, with 6/10 being assigned a conclusive score. Of these 6, one patient had a class V injury, 3 patients were diagnosed with class IV injuries, and 1 patient each was diagnosed with class III and II injuries, respectively. A total of 13 presurgical MRN reports were evaluated for these 10 patients. Eight patients were imaged on 3T scanners, and 2 were imaged on 1.5T scanners, using the same protocols. On presurgical MRN, an NS-RADS Injury (I) score was assigned to all reports except 2, which were initially inconclusive and were subsequently revised by the same expert radiologist following re-assessment blinded to the surgical findings (NS-RADS I-3/4 changed to NS-RADS I-4, NS-RADS I-2/3 changed to NS-RADS I-4). Nerve injury on presurgical MRN ranged from NS-RADS I-4 to NS-RADS I-5 injuries, with NS-RADS I-4 injuries being the most common and presenting in 8/10 patients (Figs 2–6). No patients demonstrated tongue muscle atrophy on imaging, but 1 patient was noted to have salivary gland atrophy on the ipsilateral side of the injury on MRN.
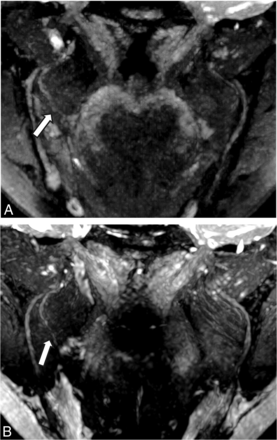
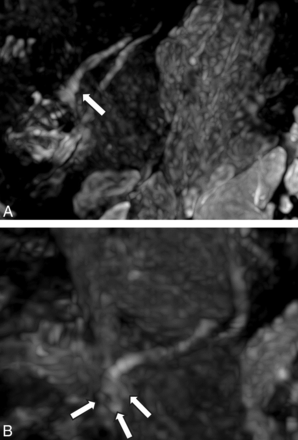
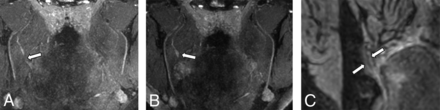
NS-RADS PI-1. A 54-year-old woman, status post third molar removal with decreased sensation, burning pain, and dysgeusia. A, Presurgical coronal MRN MIP image 89 days status post inciting event shows a neuroma in continuity of the right lingual nerve (NS- RADS I-4, arrow). B, Postsurgical coronal corresponding MRN 351 days following right lingual nerve neuroma excision and neurorrhaphy with allograft and Axoguard placement demonstrates the expected postsurgical appearance of the nerve (NS-RADS PI-1) with no loss of continuity, neuroma reformation, or substantial nerve-caliber changes (arrow).
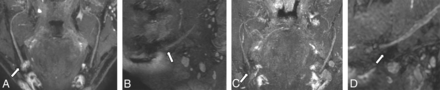
NS-RADS PI-3. A 64-year-old man with a history of multiple nerve-repair procedures of the inferior alveolar nerve and mental nerve with a history of burning pain, lip biting, and speech difficulties. A and B, Presurgical coronal and sagittal MRN MIP images demonstrate a right mental nerve lateral neuroma in continuity (NS-RADS I-4, arrows). C and D, Postsurgical coronal and sagittal MRN MIP images 353 days following neuroma excision and neurorrhaphy with allograft and Axoguard placement show a recurrent right mental nerve neuroma in continuity (arrows).
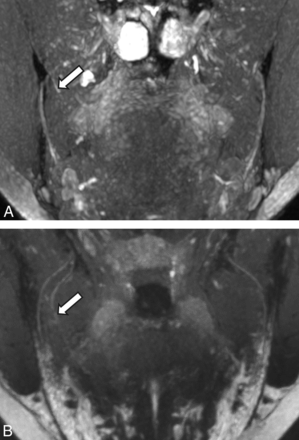
NS-RADS PI-2. A 27-year-old woman 214 days status post third molar removal with decreased sensation, burning pain, and hypogeusia. A, Presurgical coronal MRN MIP image shows the right lingual nerve demonstrating nerve-caliber focal thickening and increased signal instead of uniformly distally decreasing nerve caliber, consistent with a neuroma in continuity (NS-RADS I-4, arrow). B, Postsurgical coronal MRN MIP image 98 days status post right lingual nerve neuroma excision and neurorrhaphy with allograft and Axoguard placement demonstrates incomplete regeneration, ie, minimal increased residual signal of the nerve without a new neuroma with minor caliber change compared with preoperative MRN, consistent with NS-RADS PI-2 findings (arrow).
NS-RADS PI-3. A 21-year-old woman status post third molar removal experiencing decreased sensation, burning pain, and hypogeusia. A, Presurgical sagittal MRN MIP 55 days status post third molar removal shows right lingual nerve neuroma (NS-RADS I-4, arrow). B, Postsurgical sagittal MRN MIP 166 days status post neuroma excision and neurorrhaphy with allograft and Axoguard placement demonstrates the re-formation of multiple neuromas (arrows).
NS-RADS PI-2. A 31-year-old man status post third molar removal experiencing decreased sensation and ageusia. A and B, Presurgical coronal MRN MIP 54 days status post third molar removal shows a right lingual nerve end-bulb neuroma with complete transection with no distal continuity (NS-RADS I-5, arrows). C, Postsurgical coronal MRN MIP 238 days status post neuroma excision and neurorrhaphy with allograft and Axoguard placement demonstrates partial regeneration (arrows).
All 10 patients underwent surgery following their presurgical clinical and MRN evaluations; these procedures included microdissection with neurolysis and neuroma excision in 2 patients, neuroma excision in 2 patients, and neurorrhaphy with allograft and Axoguard Nerve Protector (Axogen) in the 6 remaining patients. A conclusive nerve injury classification was described in each surgical case, with 8 demonstrating class IV injuries and 2 showing class V injuries. The mean time from the inciting event to surgery for patients with a single inciting event was 6.1 (SD, 4.6) months. The remaining 2 patients with multiple surgeries between the inciting event and the evaluated nerve reconstruction surgery in this report had a duration of 54 and 242 months, respectively. The mean time from presurgical MRN to surgery was 46.4 (SD, 28.0) days, with a range of 11–86 days.
There were 13 postsurgical MRNs (all performed on 3T scanners) for 10 patients. These were assessed by the same expert radiologist blinded to patient outcomes. Of these, 3 patients had postsurgical MRN findings consistent with NS-RADS PI-1 (Fig 2), 3 patients had findings consistent with NS-RADS PI-2 (Figs 4 and 6), and findings of 4 were consistent with NS-RADS PI-3 (Figs 3 and 5). The mean time from surgery to postsurgical MRN was 186.5 (SD, 103.0) days, with a range of 68–353 days.
The mean postsurgical follow-up of patients was 360.2 (SD, 249.3) days, ranging from 97 to 832 days. The postsurgical clinical status of each patient is described in Table 2.
Postsurgical MRN NS-RADS PI distribution among 10 patients with the number of patients experiencing clinical improvement in specified neuropathic symptoms and overall clinical outcome
Of the 3 patients who had postsurgical MRN findings consistent with NS-RADS PI-1, two experienced partial improvement and 1 experienced complete resolution of the clinical symptoms. Of the 3 patients with NS-RADS PI-2 findings, one experienced no postsurgical change in clinical symptoms and 2 demonstrated partial improvement. One patient’s improvement was limited to lip biting and speech, with no changes in pain, sensation, or taste, while the other had no pain on initial presurgical presentation and demonstrated no improvement in tongue or taste sensation. No postsurgical change was noted in 3 of 4 patients with NS-RADS PI-3 findings, with the final patient demonstrating partial improvement in lip biting and speech challenges with, however, no sensation improvement or taste changes. Specific improvement for each clinical finding is further described in Table 2.
DISCUSSION
This is the initial report of its kind to evaluate the utility of MRN in the postsurgical patient population following nerve-repair procedures on small nerves of the jaw. With the current clinical management of such patients in the postsurgical setting being restricted to mostly clinical and NST findings, this report demonstrates a meaningful precedence for its utility and further exploration in larger or multicenter studies.
Similar to how MRN has demonstrated utility in aiding in an early diagnosis of PTNs, this clinical report demonstrates its promising potential in the setting of nerve-repair recovery. Postsurgical MRN examinations were not degraded by surgical changes and consistently demonstrated both healing and worsening structural changes following nerve-repair surgery. These pertinent MRN findings included the identification of expected postsurgical findings and pathology recurrence in the form of neuroma reformation, persistent nerve discontinuity, or perineural fibrosis. These initial MRN results additionally demonstrated a good concordance with the clinical outcomes experienced by patients. This result was because all 3 patients with NS-RADS PI-1 findings experienced partial improvement or complete resolution, whereas only 1 of 3 patients with NS-RADS PI-2 and 1 of 4 patients with NS-RADS PI-3 findings had similar improvement. Of the 3 patients who demonstrated NS-RADS PI-1 findings, all 3 experienced a concordant improvement in sensation and pain and 2 demonstrated improvement in taste sensation as well. Of interest, lip biting and speech difficulties appeared to improve regardless of the postsurgical MRN findings. This result may be because mechanical issues could improve with behavioral and speech therapies, though we did not have the full details of such treatments in the patients’ charts. The somatic and special sensory responses seem to be more resistant to optimal improvement.18
The need for follow-up MRN analysis is encouraged by our findings that showed good corroboration with the clinical outcomes. Just as initial nerve injuries have a spectrum of outcomes, nerve-repair surgical outcomes often vary due to the severity of initial injury, the complexity/invasiveness of the procedure, and the subtle complexities of healing inherent in each patient. These challenges have been described in multiple nerve-repair clinical outcome reviews and further substantiate the need for improved visualization of the nerve-healing processes.19⇓⇓-22 This initial report demonstrates the promising ability of MRN to fill this need and aid in the clinical decision-making of PTN management.
In this early analysis of the utility of MRN in the postsurgical setting, an obvious limitation is the small case sample size. This is, in part, due to MRN being a relatively novel imaging technique, requiring unique radiologic training, which is not widespread among practicing radiologists. Although presurgical MRN has been increasingly used and shown to correlate well with both clinical NST and surgical findings,1 postsurgical MRN has not been routinely implemented. When used, postsurgical MRN is mostly used when the desired postsurgical results are not achieved.
Although nerve injuries of varying Sunderland classifications were analyzed in this report, there is a demonstrated shift toward more severe injuries (classes IV and V). This shift is expected because these injuries are more likely to require surgical intervention as opposed to milder injuries.
This report is an initial description of postoperative MRN results. Further analysis is needed to evaluate the generalizability of these findings. Additionally, more analysis is needed to assess postsurgical MRN utility in less-severe nerve injuries, though this is likely to be a persistent challenge given current recommendations for surgical interventions in the domain of peripheral nerve injuries. With a larger sample size, additional evaluation of the timeline should also be evaluated to better determine the capacity for MRN to elicit early postsurgical structural changes and furthermore determine a suggested timeframe for imaging and related management recommendations.
Acknowledgments
This work was partly supported by Stephen B. Milan Award from the Oral and Maxillofacial Surgery Foundation.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received September 11, 2023.
- Accepted after revision December 20, 2023.
- © 2024 by American Journal of Neuroradiology