Abstract
BACKGROUND AND PURPOSE: The characteristics of large vessel occlusion (LVO) in the acute phase of pediatric arterial ischemic stroke and their natural history according to stroke etiology are poorly explored. This studied aimed at describing the prevalence and the radiological evolution of LVO in pediatric AIS.
MATERIALS AND METHODS: This single-center retrospective study included consecutive non-neonate children with acute arterial ischemic stroke, intracranial proximal LVO in the anterior circulation (MCA, anterior cerebral artery, and/or ICA), and clinical and imaging follow-up for at least 18 months, during a 9-year period.
RESULTS: Intracranial LVO was observed in 24.8% of patients with anterior circulation arterial ischemic stroke and adequate follow-up (n = 26/105), with a median age of 4.2 years (IQR 0.8–9), sex ratio 1.16. The main stroke etiology associated with LVO was unilateral focal cerebral arteriopathy (n = 12, 46%). During follow-up, a specific pattern of unilateral poststroke anastomotic bridge was observed in 8/26 patients, with the poststroke development of nonperforating collaterals forming a bridge in bypass of the LVO site with visible distal flow, within a median delay of 11 months. The development of unilateral poststroke anastomotic bridge was only observed in patients with unilateral focal cerebral arteriopathy. No patient with this pattern experienced stroke recurrence or further progressive vascular modifications.
CONCLUSIONS: After stroke, the development of unilateral poststroke anastomotic bridge is specifically observed in children with focal cerebral arteriopathy, appearing in the first year after stroke. This clinical-radiologic pattern was not associated with stroke recurrence or arterial worsening, differentiating it from progressive intracranial arteriopathy, such as Moyamoya angiopathy.
ABBREVIATIONS:
- ACA
- anterior cerebral artery
- AIS
- arterial ischemic stroke
- FCA
- focal cerebral arteriopathy
- IQR
- interquartile range
- LVO
- large vessel occlusion
- MMA
- Moyamoya angiopathy
- MT
- mechanical thrombectomy
- PCA
- posterior cerebral artery
- U-PS
- unilateral post stroke
Stroke in children is a rare but devastating event, with long-lasting developmental consequences affecting the child’s and family’s lives. Large vessel occlusion (LVO) is observed in 20%–25% of pediatric arterial ischemic stroke (AIS)1,2 and there is growing evidence for the use of mechanical thrombectomy in this setting to improve long-term outcomes.3⇓⇓⇓⇓⇓-9 However, there is still some debate concerning the importance of including stroke etiology in the decision-making process, as focal cerebral arteriopathy (FCA) seems to be associated with an increased rate of re-occlusion after mechanical thrombectomy.8 Indeed, the specificities of LVO according to stroke etiology in children and the angiographic natural history of pediatric LVO have not been reported so far. This study explored LVO characteristics in pediatric AIS according to stroke etiology, specifically addressing radiologic and clinical outcomes with and without recanalization treatments, focusing on poststroke collateral vessel development.
MATERIALS AND METHODS
This study is a retrospective analysis of the prospectively maintained single-center database of pediatric AIS in the Necker-Enfants Malades University Hospital, Paris, France. Patients were included if they fulfilled the following criteria: 1) non-neonate pediatric patients (28 days – 17 years old) presenting with AIS in the anterior circulation between January 1, 2010, and December 31, 2018; 2) large vessel occlusion in the intracranial anterior circulation, eg, proximal middle cerebral artery (MCA, M1, and/or M2 segments) and/or the proximal anterior cerebral artery (ACA, A1 segment) and/or the terminal portion of the ICA observed on imaging at the acute phase; and 3) available clinical, parenchymal, and vascular imaging follow-up for at least 18 months. Clinical and demographic data, medical history, and etiological work-up results were extracted from the local database. Imaging data (MR, CT, MRA, CTA) were centrally reviewed. Stroke characteristics and vascular findings at the acute phase and during follow-up were recorded. Stroke recurrence was defined as persistent new focal deficit associated with a new ischemic lesion on MR imaging after initial stroke. Early vessel reocclusion without new ischemic lesion, or TIA were not considered as a stroke recurrence. Retained stroke etiology was determined according to the CASCADE classification for pediatric stroke (Online Supplemental Data).10
Because of the small sample, data were expressed in median and interquartile range (IQR). Comparisons of values between subgroups used nonparametric tests, eg, Wilcoxon rank sum test. Participants’ legal guardians provided consent for the anonymized use of data.
RESULTS
Prevalence of Anterior Circulation LVO
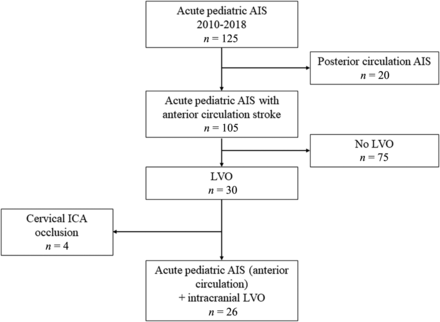
During the inclusion period, 105 patients aged 28 days to 17 years old had both an AIS in the anterior circulation territories and available clinical and imaging follow-up for at least 18 months. Among them, intracranial LVO was observed in 26 patients (24.8%), 14 boys and 12 girls, with a median age of 4.2 years old (IQR, 0.8–9) (Fig 1).
Study flow chart.
Characteristics of LVO and Stroke at the Acute Phase
LVO was determined on TOF-MRA for all patients, including 2 patients who had both MRA and CTA at the acute phase. Intracranial LVO was unilateral in 25/26 patients, more frequent on the left side (n = 16, right side n = 9, P = .019). The most frequent site of occlusion was the proximal MCA (n = 25, M1 segment n = 16, M2 segment n = 9) and 9/26 patients had a large occlusion involving several arterial segments. LVO was associated with stenosis of other intracranial arteries in 8/26 patients (homolateral n = 7, contralateral n = 1). Brain infarction involved deep MCA territory (n = 20), superficial MCA territory (n = 21), and/or ACA territory (n = 3). Most patients (17/26) had no previous medical history. The other patients had a cardiac condition (malformative shunting cardiopathy n = 3, cardiac failure with extracorporeal membrane oxygenation support n = 1), sickle cell anemia (n = 1), leukemia (n = 1), systemic disease (FARSA deficiency,11 n = 1), or recent benign head trauma (n = 1). A significant proportion of patients (n = 10/26, 38%) received a hyperacute recanalization treatment, including mechanical thrombectomy (n = 3). Final etiological diagnosis of AIS according to the Childhood AIS Standardized Classification And Diagnostic Evaluation (CASCADE) classification showed a predominance of intracranial arteriopathy (n = 15/26). Retained etiologies were the following: unilateral FCA (n = 12, 46%, all of FCA-i [infectious/inflammatory) type], bilateral cerebral arteriopathy of childhood (n = 3, 11%), cervical/aortic arteriopathy (n = 3, 11%), cardioembolic (n = 7, 27%), hematologic/thrombotic (n = 1, 4%). Patients with FCA were younger than patients with other stroke causes (median age 1.93 versus 6.33 years old, P = .06) (Online Supplemental Data).
Clinical and Angiographic Outcomes
Median follow-up duration was 31 months (IQR 18–38), with a median 4 arterial imaging procedures per patient during the study period (8 CTA angio-CTs, 111 MRAs). The ratio of patients with vessel patency (complete patency or residual stenosis) slightly increased over time on poststroke serial imaging: 50% in the 12 patients re-imaged ≤48 hrs after stroke diagnosis/treatment (n = 6, of which 4 had received IV r-tPA and 1 IV r-tPA and mechanical thrombectomy), 58% in the 24 patients imaged at 1–3 months after stroke, and 62.5% in the 24 patients imaged at 12–18 months after stroke. A stroke recurrence was noted in 6/26 patients (23%) during follow-up, whose stroke etiologies according to CASCADE classification were cardio-embolic (n = 3), aortic/cervical arteriopathy (n = 1), bilateral cerebral arteriopathy of childhood/Moyamoya angiopathy (MMA) (n = 1), and hematologic/thrombotic (n = 1).
Poststroke Development of Collateral Vessels
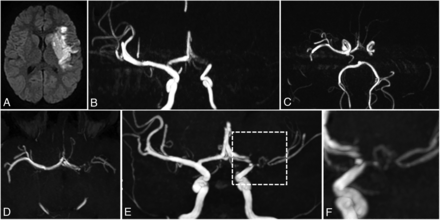
During follow-up, collateral vessels originating from the occluded/stenotic arterial segment were observed in 11/26 patients. Collateral vessels were already present on the acute phase imaging in 2 patients, fulfilling the diagnostic criteria for MMA.12 For 9 patients without observed collaterals at the acute phase, a progressive development of such vessels was noted during follow-up (Online Supplemental Data). One patient with bilateral intracranial arteriopathy developed further bilateral collaterals and had a MMA diagnosis. The 8 patients with initial strictly unilateral intracranial artery involvement who developed further homolateral collaterals displayed remarkable common temporal and anatomic characteristics. These collateral vessels had a typical pattern forming a bridge in bypass of the residual stenosis or occlusion, starting upstream from the arterial stenosis/occlusion, spanning over the stenotic/occluded zone, and connecting downstream with the main arterial trunk with visible distal flow. They did not display a perforating lenticulostriate route, but they were parallel to the main trunk of the originating artery (MCA n = 7 and/or ACA n = 3) though distinct from it. They were thin and sometimes serpiginous, without draining veins. This specific aspect was thus labeled unilateral poststroke (U-PS) anastomotic bridge (Fig 2). Median development delay of U-PS anastomotic bridge was 11 months (IQR 5–12). After the development of this U-PS anastomotic bridge, the vascular aspects of the circle of Willis and of the U-PS anastomotic bridge itself were stable over the rest of the follow-up period (Fig 3). Of note, no patient with U-PS anastomotic bridge had stroke recurrence during follow-up.
U-PS anastomotic bridge development in a patient with AIS and LVO, and unilateral FCA. Upper panel: acute phase MR imaging, showing recent MCA infarction with DWI hypersignal (A), with proximal left MCA occlusion and left A1 stenosis on the time-of-flight MRA, coronal view MIP 15 mm (B) and axial view MIP 10 mm (C). No collateral is visible at the acute phase. Lower panel: MR imaging 12 months after stroke occurrence. Time-of-flight MRA shows strictly unilateral left anomalies. Axial view (D) shows a reverted to normal left A1 segment and a persisting steno-occlusive M1 lesion, favoring the diagnosis of FCA. E, Coronal view shows a U-PS anastomotic bridge bypassing the M1 occlusion, with distal visible flow. F, Closer view of the U-PS anastomotic bridge illustrates the collaterals direction, parallel to the main MCA trunk, without perforating lenticulostriate collaterals.
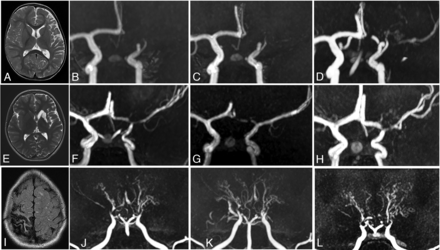
Patterns of angiographic evolution in patients with FCA and U-PS anastomotic bridge development, compared with Moyamoya angiopathy. Upper panel: Patient with left superficial MCA infarction (A, MRI axial T2) and FCA. Angiographic evolution (3D time-of-flight MRA) with persisting occlusion 6 and 12 months after stroke (B and C), and U-PS anastomotic bridge in bypass of the occluded M1 segment with visible downstream MCA segments (D). Middle panel: Patient with left deep and superficial MCA infarction (E, MRI axial T2) and FCA. Angiographic evolution (3D time-of-flight MRA) with initial M1 occlusion (F). Partial improvement of MCA 6 months after stroke (G), with the observation of U-PS anastomotic bridge 12 months after stroke (H). Lower panel: Patient with right superficial MCA infarction (I, MRI axial T2) and Moyamoya angiopathy. Angiographic evolution (3D time-of-flight MRA) with bilateral steno-occlusive lesions of the terminal ICAs, MCAs, and ACAs (J). Bilateral perforating collaterals, present at stroke onset (J) and developing over time with a classical puff of smoke appearance. Progression of the arteriopathy with disappearance of MCAs and ACAs 6 and 24 months after stroke (K and L).
Interestingly, the development of U-PS anastomotic bridge was only observed in patients with unilateral FCA as a stroke cause (n = 8/8). In patients with unilateral FCA and LVO, 8/12 (66.7%) developed U-PS anastomotic bridge. U-PS anastomotic bridge development was not associated with age at stroke onset, stroke location, severity, or management, including recanalization treatments (Online Supplemental Data). Furthermore, U-PS anastomotic bridge development was not different in patients with FCA with persisting LVO and in patients with FCA with vessel patency evaluated at 3 months after stroke (n = 3/5 versus n = 5/7, not significant).
The 3 patients with bilateral collaterals had a very different course. They had bilateral cerebral arteriopathy, and 2 of them had visible collaterals at stroke onset imaging, with a perforating lenticulostriate route, consistent with Moyamoya network. One patient developed poststroke collaterals associating both Moyamoya network and U-PS anastomotic bridge. These patients met the diagnostic criteria for MMA. Two had arterial worsening after stroke and 1 experienced a stroke recurrence during follow-up.
DISCUSSION
This study addressing anterior circulation LVO in pediatric AIS (excluding neonates) provides novel data concerning a specific radiologic evolution pattern of intracranial LVO in children relating to stroke etiology.
In our series, intracranial LVO was observed in 24.6% of children with acute anterior circulation AIS. Though our study focused only on anterior circulation LVO, these findings are in line with the reported prevalence of LVO in childhood stroke of 23.5% in a retrospective population-based cohort study by Bhatia et al2 and 22.4% in a single-center retrospective study by Bonnet et al.1
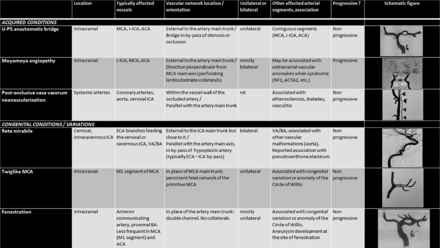
Concerning angiographic outcomes, we described a specific pattern of U-PS anastomotic bridge, characterized by the development in the first year after stroke of a collateral network forming a bridge in bypass of the residual stenosis or occlusion, with visible distal flow in the still or formerly occluded main arterial trunk, in patients without visible collaterals at the acute phase. This pattern is specific as it does not meet the criteria for other described approaching vascular patterns (Fig 4).
Distinctive features of U-PS anastomotic bridge compared with vascular patterns with close appearance. (i)-ICA = (intracranial)-internal carotid artery; ECA = external carotid artery; VA = vertebral artery; BA = basilar artery. Illustration is from Lin et al22 for rete mirabile. Drawings by F.B. and M.K., with courtesy.
First, as collaterals were not present at stroke onset, this precludes congenital variations or anomaly, such as fenestration, duplication, aplastic or twig-like artery aspects. In these congenital variations, the regression of anastomoses from the embryonic primitive mesh of intracranial arterial networks did not occur, leading to the persistence of remnants of embryologic development.13⇓⇓-16 They are sometimes reported as a potential risk factor for ischemic or hemorrhagic stroke.17,18 Of note, no such variation was observed in our patients. Second, as U-PS anastomotic bridge developed after LVO, a specific intracranial arterial compensatory mechanism of downstream chronic ischemia can be hypothesized. Indeed, a similar compensatory mechanism has been described in cervical or systemic arteries, occurring during the embryonic phase, or acquired hypoxic conditions. Cervical carotid rete mirabile is an embryonic compensatory phenomenon, visible as a meshwork of multiple, freely intercommunicating arterioles fed by external carotid artery branches, which reconstitute the absent or hypoplastic segments of the internal carotid artery. It may be bilateral and associated with other vascular aspects (aorta malformation, posterior circulation rete mirabile) or a general condition (pseudoxanthoma elasticum).19⇓⇓-22 It may represent a risk factor for ischemic or hemorrhagic cerebrovascular diseases.23 Postocclusive neovascularization has been described in systemic arteries, in which vasa vasorum, adventitial vessels form plexus in the wall of large blood vessels, with a mainly nutritive role; development would be stimulated by local subacute hypoxic conditions (atherosclerosis, diabetes, vasculitis, etc), figuring a phenomenon of compensatory neovascularization. This has been notably reported in coronary arterys, aorta, and cervical internal carotid artery occlusion.24⇓-26 To our knowledge, these mechanisms have not been reported affecting the circle of Willis, except in a handful of case reports.27 The existence of intracranial vasa vasorum has been debated, and they are suggested to be present only in the proximal parts of MCA, ACA, and intracranial ICA.28⇓-30 U-PS anastomotic bridge could thus represent a similar mechanism of postocclusive neovascularization, with the development of intracranial vasa vasorum located in the proximal segments of the circle of Willis arteries.
Interestingly, not every patient with LVO developed U-PS anastomotic bridge. No association with age at stroke onset or duration of vessel occlusion was found. U-PS anastomotic bridge development was strongly associated with stroke etiology: this pattern was only observed in patients with unilateral FCA, and 66% of children with FCA and LVO developed U-PS anastomotic bridge. This suggests that, rather than a nonspecific mode of vascular healing after local ischemic changes related to LVO, it could be related to subacute local conditions and modifications associated with FCA, for instance, subacute hypoxia and/or vessel wall inflammation, stimulating angiogenic factors.30,31
Finally, it seems important to differentiate this vascular aspect from Moyamoya Angiopathy MMA. MMA is a progressive steno-occlusive disease involving the distal internal cerebral artery and its bifurcation, and the adjacent proximal ACA and MCA. It often shows bilateral, symmetric, or asymmetric segmental stenoses of the involved arteries. Stenoses are associated with several types of developing compensatory vessels: basal collateral vessels, leptomeningeal collaterals, and anastomotic internal-external carotid systems collaterals. Basal collaterals are abnormally dilated lenticulostriate and thalamo-perforating arteries, arising from the MCA to the basal ganglia and thalamus.12,27,32 Key points differentiate MMA and U-PS anastomotic bridge: 1) in MMA dilated perforating arteries supplying the basal ganglia arise perpendicularly from the MCA trunk, whereas in U-PS anastomotic bridge collaterals go parallel with the MCA main trunk (the difference is well observed on coronal view) (Figs 3 and 4); and 2) MMA is classically bilateral, sometimes asymmetrical but rarely unilateral, whereas U-PS anastomotic bridge is unilateral without contralateral arterial anomaly (stenosis/occlusion). The latter point is of utmost importance as both an aspect of anastomotic bridge and abnormal perforating lenticulostriate (Moyamoya network) may be observed in patients with Moyamoya. It seems important to identify isolated U-PS anastomotic bridge because, though these patients have developing collaterals, they did not experience any stroke recurrence nor vascular disease progression, contrary to patients with Moyamoya angiopathy. In our study, recurrent strokes only occurred in non-FCA patients. This emphasizes the fact that patients with FCA usually do not experience recurrent stroke regardless of whether they had collaterals or not. Thus, the observation of a U-PS anastomotic bridge after stroke with an isolated strictly unilateral LVO, and no contralateral vascular anomaly should not be considered as a risk for poor outcome or stroke recurrence.
Our study limitations mainly relate to the small sample and to its retrospective nature. Different angiographic imaging modalities may also induce biases: TOF-MRA may over-call occlusion in a stenosis with minimal flow, and 3T MRA may have a better resolution than 1.5T MRA for the anastomotic bridge observation. However, the homogeneous technique in our study may limit the bias for intrasample angiographic comparisons: 25/26 patients had TOF-MRA in the acute phase and during follow-up, mostly on 1.5T scan. Only 1 patient had initial MRA and CTA at 48 h poststroke, but he had a persistent right proximal M2 segment occlusion, which precludes a bias of patency related to imaging technique.
CONCLUSIONS
LVO represented 24.8% of non-neonatal pediatric AIS in the anterior circulation in our study. A specific pattern of U-PS anastomotic bridge, with unilateral network of collaterals bypassing the affected zone, appearing in the first year after stroke and remaining stable without stroke recurrence, is strongly associated with FCA. Further studies are needed to confirm the association of U-PS anastomotic bridge with FCA, and to refine differences between unilateral FCA with collaterals and MMA.
Acknowledgments
We thank Kim Tran Dong for technical assistance.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
REFERENCES
- Received August 18, 2023.
- Accepted after revision December 1, 2023.
- © 2024 by American Journal of Neuroradiology