Abstract
BACKGROUND AND PURPOSE: Despite its rarity in Western countries, kernicterus resulting from severe neonatal hyperbilirubinemia and its associated neurologic consequences still persists. Subtle MR imaging patterns may be overlooked, leading to diagnostic and prognostic uncertainties. The study systematically analyzes MR imaging pattern over time.
MATERIALS AND METHODS: A retrospective MR imaging study was conducted in Departments of Pediatric Neurology at the University Children's Hospitals in Leipzig, Germany, or Tübingen, Germany, between 2012 and 2022 in patients who presented beyond the neonatal period suspected of having chronic kernicterus.
RESULTS: Eight patients with a total of 15 MR images were identified. The clinical diagnosis of kernicterus was confirmed in all cases on the basis of typical MR imaging findings: Bilateral, diffuse hyperintensity of the globus pallidus was observed in the neonatal period on T1WI (1 MR imaging, at 2 weeks), in infancy on T2WI (4 MR images, at 9–26 months). In children 2 years of age and older, bilateral hyperintensity on T2WI was limited to the borders of the globus pallidus (8 MR images, at 20 months –13 years). Notably, 2 children exhibited normal initial MR imaging findings at 2 months of age. Hence, MR imaging depiction of kernicterus pathology evolves with time, first evident on T1WI, subsequently on T2WI, with a “blind window” during early infancy. The T2WI signal change initially involves the entire globus pallidus and later is limited to the borders. Kernicterus had not been diagnosed in any except 2 patients by previous investigators.
CONCLUSIONS: All patients presented with a characteristic clinical history and signs and an evolving MR imaging pattern. Nonetheless, the diagnosis of kernicterus was frequently missed. Abnormalities on later MR images appear to be underrecognized.
ABBREVIATIONS:
- ABE
- acute bilirubin encephalopathy
- BFMF
- Bimanual Fine Motor Function
- CP
- cerebral palsy
- GMFCS
- Gross Motor Function Classification System
- GP
- globus pallidus
In neonates, the physiologic increase of unconjugated bilirubin typically occurs around days 5–7. Bilirubin, being lipophilic, can be freely distributed throughout all tissues. This is not problematic as long as the increase is moderate. However, at high concentrations, deposition occurs in various tissues, especially in the basal ganglia, hippocampus, cerebellum, and cranial nerve nuclei. Failure to initiate appropriate therapy such as phototherapy or exchange transfusion may result in bilirubin deposition causing damage to vulnerable brain structures (basal ganglia and brainstem nuclei), leading to the clinical manifestations of kernicterus.1,2 Symptoms of acute bilirubin encephalopathy (ABE) include lethargy, muscular hypotonia, feeding problems, high-pitched crying, retrocollis, fever, apnea, and seizures. Chronic kernicterus refers to the residual damage that occurs following the acute phase. It is a spectrum disease, depending on the severity, duration, and timing of exposure to unconjugated bilirubin.3 Long-term sequelae commonly observed are dyskinetic cerebral palsy (CP) with varying degrees of severity and hearing disorders up to complete deafness. Also, eye-movement disorders, in particular vertical gaze palsy, are typical. Dental enamel dysplasia may also develop. Historically, kernicterus accounted for approximately 10% of cases of CP, notably the dystonic or athetoid form.4
With an improved understanding of the underlying pathomechanism, bilirubin is now monitored in neonates, and prophylactic treatment with phototherapy and, in severe cases, exchange transfusions is established. Phototherapy is recommended when bilirubin levels reach ≥20 mg/dL after 72 hours of life. If the neonate has an infection, is premature, or has an early and rapid rise in bilirubin, treatment is indicated at lower levels. These interventions have dramatically reduced the incidence of kernicterus. In countries with low infant mortality, the incidence was estimated to be 0.4–2.7 per 100,000 between 1988 and 2005.5
However, kernicterus has not disappeared completely. Hemolytic disorders such as Rh or ABO incompatibility or predisposing underlying diseases associated with excessive hyperbilirubinemia in the neonatal period, eg, glucose-6-phosphate dehydrogenase deficiency and Crigler-Najjar syndrome, can lead to kernicterus if not rigorously treated. In addition, critically ill neonates with sepsis and hypoalbuminemia or preterm-born children are at higher risk. Monitoring bilirubin in neonates may be insufficient. In Western countries, this is possibly due to neglect because the disease has lost its terror and the consequences are no longer present. Early hospital discharge, breastfeeding, and negligent neonatal monitoring contribute to an increased risk.6⇓⇓⇓-10 In developing countries, comprehensive monitoring of neonates may not be widely established, resulting in a higher incidence of untreated hyperbilirubinemia. The availability of therapies and the decline in incidence have led to a reduced awareness of kernicterus and its diagnostic criteria.6
In MR imaging, the typical pattern observed in kernicterus is bilateral hyperintensity in the globus pallidus (GP), which varies with time. This characteristic finding is not always prominent and can be easily overlooked. During the acute phase in the neonatal period, the GP demonstrates a bilateral signal increase on T1WI, while in the chronic phases, the signal increase is observed on T2WI.11 Additionally, there may be a bilateral signal increase in the subthalamic nucleus and volume loss in the hippocampus.12
The diagnosis of chronic kernicterus signifies an acquired and nonprogressive encephalopathy and enables counseling regarding future development.
This study aimed to retrospectively analyze MR imaging findings of patients clinically suspected of having chronic kernicterus, with a focus on changes in the GP signal.
MATERIALS AND METHODS
A retrospective MR imaging study was conducted on patients who presented beyond the neonatal period and were suspected of having chronic kernicterus in the departments of Pediatric Neurology at the University Hospital for Children and Adolescents in Leipzig, Germany, or Tübingen, Germany, between 2012 and 2022. The primary purpose of their hospital visits was to diagnose developmental and movement disorders.
The study adhered to ethical guidelines and obtained proper consent (AZ 330–13-18112013). It followed the standards of good clinical practice and the Declaration of Helsinki.
The inclusion criteria for patients were as follows (Tables 1 and 2):
The presence of dyskinetic CP characterized by involuntary, uncontrolled, recurring, occasionally stereotyped movements; fluctuating muscle tone; and persistent primitive reflex patterns
Developmental delay with an onset in the neonatal period
A history of hyperbilirubinemia in the neonatal period
Hearing impairment, vertical gaze paresis, and dental enamel dysplasia were documented but were not mandatory inclusion criteria.
Absolute inclusion and exclusion criteria
Relative inclusion criteria
The following criteria were used to exclude patients:
Movement disorders with onset after infancy
Progressive disorders such as neurometabolic diseases
Other explanatory causes such as peripartum asphyxia; congenital brain anomalies like migration, gyration, and hypomyelinating disorders; and genetic diseases
No history of hyperbilirubinemia in the neonatal period.
MR imaging analyses were performed by 2 authors (T.N. and I.K.-M.). Analysis was initially conducted independently; then, a consensus was reached for the final allocation.
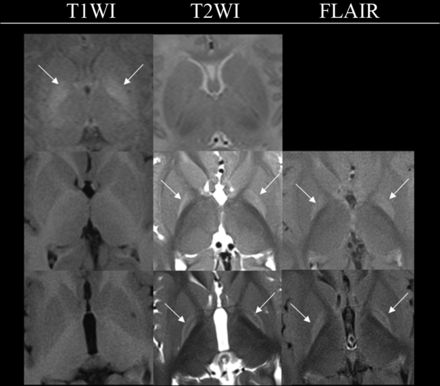
Signal changes in the GP were classified into the following categories (illustrated in Fig 1):
Pattern I: diffuse signal increase on T1WI
Pattern IIa: diffuse signal increase on T2WI and/or FLAIR
Pattern IIb: signal increase on T2WI and/or FLAIR involving only borders.
Examples illustrating the various patterns observed with respect to bilateral signal change in the GP in kernicterus. The upper row displays pattern I, characterized by a diffuse signal increase on T1WI (arrows), while T2WI shows no abnormalities (sample case one, 2 weeks). The middle row represents pattern IIa, where T1WI appears normal, but there is a diffuse signal increase on T2WI (arrows) and FLAIR (arrows), (sample case three, 11 months of age). The lower row shows pattern IIb, featuring normal T1WI findings and a signal increase on T2WI (arrows) and FLAIR (arrows), limited to the borders (sample case two, 2 years 10 months of age).
Additional changes were noted as either present or absent in the following areas: hippocampal structures (including signal change or atrophy defined as enlarged surrounding spaces) and other brain areas as specified.
RESULTS
A total of 8 patients (followed up for 6–13 years) were included in the study, and a total of 15 MR imaging examinations were performed. All patients had at least 1 MR image, 6 patients had 2, and 1 patient had 3 MR images (Online Supplemental Data).
Case 1
Patient 1 developed hyperbilirubinemia with a maximum bilirubin level of 30 mg/dL because of glucose-6-phosphate dehydrogenase deficiency and was treated by phototherapy. At 10 years of age, he showed mild dyskinetic CP (Gross Motor Function Classification System [GMFCS I], Bimanual Fine Motor Function [BFMF II], Viking Speech Scale II).
Case 2
Patient 2 had ABE diagnosed at day 6 after birth. His bilirubin level was 36.4 mg/dL. He underwent phototherapy, exchange transfusion, immunoglobulin infusion, and anticonvulsant therapy with phenobarbital. He developed epilepsy beyond the neonatal period. The clinical examination at 6 years of age revealed dyskinetic CP (GMFCS V, BFMF V, Viking Speech Scale IV), vertical gaze palsy, enamel dysplasia, deafness, and dysphagia.
Case 3
The child was born as a twin at 33 + 6 weeks of gestation with a birth weight of 1490 g. On day 2, phototherapy was started; the maximum bilirubin level was 11.7 mg/dL. During the first days, hypoalbuminemia was noticed (2.4 mg/dL). At 7.5 years of age, he had dyskinetic CP (GMFCS V, BFMF V, Viking Speech Scale IV). A severe hearing loss was treated with a cochlear implant.
Case 4
A girl born at 34 + 5 weeks of gestational age developed necrotizing enterocolitis and subsequent sepsis postpartum. The bilirubin level increased to 25 mg/dL when phototherapy was started (though phototherapy is recommended in such a situation, prematurity and sepsis, at a bilirubin level of ≥12 mg/dL). Clinical examination at 13 years of age showed dyskinetic CP (GMFCS V, BFMF V, Viking Speech Scale IV), vertical gaze palsy, and deafness treated with hearing aids.
Case 5
The girl was born in Pakistan at 36 weeks of gestation. A few days postpartum, she developed ABE. The bilirubin level was not measured. At 6 years of age, she had dyskinetic CP (GMFCS IV, BFMF IVa, Viking speech scale IV), vertical gaze palsy, and deafness.
Case 6
The patient was born preterm at 35 weeks of gestational age and had severe neonatal sepsis. His bilirubin level increased to 25 mg/dL, so he was treated by exchange transfusion. The clinical examination at 11 years of age revealed mild dyskinetic CP (GMFCS I, BFMF II, Viking Speech Scale II), vertical gaze palsy, and hearing loss treated with a cochlear implant.
Case 7
The boy was born in Iran. He had ABE with a hyperbilirubinemia level of 29 mg/dL. Phototherapy and exchange transfusion were performed. Clinical findings at 6 years of age revealed severe dyskinetic CP (GMFCS V, BFMF V, Viking speech scale IV), recurrent dystonic crises, vertical gaze palsy, and dysphagia.
Case 8
The boy was born in Afghanistan and developed ABE on the fourth day of life. Clinical examination at age 11.5 years revealed severe dyskinetic CP (GMFCS V, BFMF V, Viking Speech Scale IV), vertical gaze palsy, dental enamel hypoplasia, and deafness. Due to dysphagia, he developed cachexia.
According to the clinical criteria, the diagnosis of kernicterus could be made in all the above-reported patients, but only 2 had been diagnosed by the previous MR imaging investigators (patients 1 and 3). In 2 other patients (patients 4 and 5), abnormalities in the GP were noticed (patient 5 only on the second MR imaging), but these findings were not assigned to kernicterus. In the initial reporting, 10 of 15 MR images had been evaluated with unremarkable findings.
A total of 15 MR images were re-evaluated using the above criteria (Figs 1 and 2). The assessment by the 2 raters did not differ. The MR images had been obtained in different hospitals (even in different countries) on different scanners (usually 1.5T) with different sequences, resulting in varying image quality.
On re-examination, 13 of the 15 MR images showed pathologic findings, characterized by a signal increase in the GP observed on either T1WI or T2WI. The 2 MR images considered to have unremarkable findings were obtained at 2 months of age (patients 2 and 3, the latter at the corrected age of 2 months), but subsequent follow-up at later ages revealed typical signal changes in the GP on T2WI. Thus, on the basis of the clinical criteria and the MR images, kernicterus was confirmed in all 8 patients, including the 6 patients with previously unconfirmed diagnoses based on radiology.
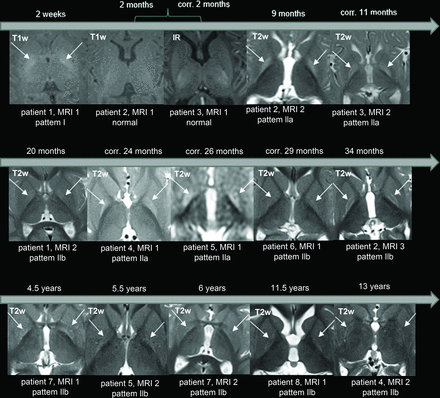
Analysis of the signal abnormalities in the GP across time revealed the following distinct evolution pattern.
Bilateral, diffuse hyperintensity on T1WI was observed in the neonatal period (1 MR image at 2 weeks). Subsequently, bilateral, diffuse hyperintensity of the GP on T2WI (pattern IIa) appeared during infancy (4 MR images at 9, 11, 24, and 26 months of age). In children 2 years of age and older, bilateral hyperintensity on T2WI was observed, specifically involving the borders of the GP (pattern IIb) (8 MR images at 20, 29, 34 months of age, 4.5, 5.5, 6, 11.5 and 13 years). Therefore, the pathology associated with kernicterus, as depicted by the MR images, changed with time, initially evident on T1WI and then on T2WI. Furthermore, considering the 2 children initially imaged at 2 months of age with normal MR imaging findings, a “blind window” in early infancy seems to exist. Notably, the signal change on T2WI initially involved the entire GP, and later, after the second year (with some overlap), it primarily affected the borders (Figs 2 and 3).
The temporal progression of the GP signal changes in 8 patients. A bilateral signal change in the GP is the characteristic sign of kernicterus. In the neonatal period, hyperintensity is observed on T1WI (arrows). However, at approximately 2 months of age, a “blind diagnostic window” is encountered, where neither T1WI nor T2WI/FLAIR show abnormal findings. Subsequently, during infancy, there is signal hyperintensity of the entire GP on T2WI/FLAIR (arrows). After around 2 years, the signal hyperintensity on T2/FLAIR is limited to the borders of the GP (arrows). IR indicates inversion recovery; corr., corrected age related to gestational age.
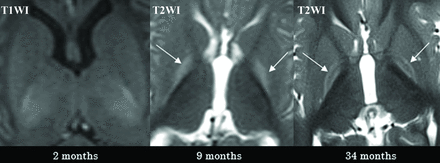
Case 2 demonstrates the typical temporal sequence in a single patient (patient 2). The initial MR imaging was performed at approximately 2 months of age, revealing no abnormalities (illustrated on T1WI, left). At 9 months of age (middle), the entire GP exhibited a diffuse signal increase on T2WI (pattern IIa, arrows). At 34 months of age (right), a signal increase on T2WI was observed solely at the borders of the GP (pattern IIb, arrows).
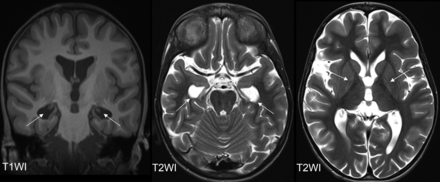
Two patients exhibited a thinned hippocampus (patients 2 and 8, Fig 4), and these cases represented the most severe manifestations. Thus, it is hypothesized that hippocampal volume loss correlates with the severity of residual damage. No further MR imaging pathologies were observed.
Hippocampal volume loss is illustrated in patient 8 at the age of 11.5 years. The coronal T1WI (left) displays the head of the hippocampus only as filiform structures (arrows), resulting in enlarged temporal horns, as also depicted in the axial T2WI (arrows, middle). Both the external and internal spaces are globally enlarged (axial T2WI, right). The right images highlights the bilateral signal changes in the GP (arrows), indicative of pattern IIb.
DISCUSSION
Kernicterus continues to be present in the Western world in neonates with predisposing diseases representing a higher risk. Additionally, the influx of patients from regions with inadequate neonatal care, where kernicterus is more prevalent, has contributed to its persistence.
Our study confirmed that the pathology of kernicterus, as observed through MR images, evolves with time. Previous literature13⇓⇓-16 has extensively reported different signal characteristics on T1WI and T2WI during the neonatal period. Coskun et al,14 in 2005, reported increased T1 in the GP as a common and characteristic finding of acute kernicterus. They observed this abnormality in 8 of 13 neonates (gestational ages, 34–40 weeks) between 5 and 25 days after birth. Okumura et al,17 in 2021, found MR imaging abnormalities in the GP less frequently in preterm infants with bilirubin encephalopathy (scanned between 36 and 41 weeks' corrected gestational age) compared with mature infants. They reported that this discrepancy may be attributed to the timing of the MR image acquisition with respect to the less clear onset of bilirubin encephalopathy in preterm infants. Wisnowski et al11 cautioned about the possibility of false-positive interpretations of high T1 signal in the neonatal period. They mentioned that abnormal T1-signal in the GP and subthalamic nucleus can be confused with “normal” T1 signal related to myelination, particularly with higher field strengths and modern 3D T1-weighted sequences that provide better SNR and increased sensitivity to pathology as well as normal developmental processes like myelination.
Apart from the known changes in the GP, we discovered that these changes progress through 4 phases: In phase 1, occurring during acute kernicterus in the neonatal period, there is a diffuse increase in signal intensity in the GP on T1WI. This is followed by a phase 2 interval in which no abnormalities are apparent on either T1WI or T2WI, creating a blind window that was observed at 2 months of age. Subsequently, in phase 3 during infancy, a characteristic diffuse hyperintensity of the entire GP is evident on T2WI. From approximately 2 years of age, with some overlap, the signal increase becomes evident only at the border of the GP in phase 4.
Yokochi,18 in 1995, described the posterior medial border of the GP as the most sensitive to bilirubin by MR imaging. This finding could explain why this area exhibits longer-lasting and probably permanent T2 signal changes on MR images, indicating significant damage.
We were able to demonstrate 2 novel aspects, to the best of our knowledge: first, the presence of a potentially blind window after the neonatal period, in which the signal changes are not apparent on either T1WI or T2WI. Second, the development of GP hyperintensity characteristics on T2WI in chronic kernicterus, with the signal changes initially involving the entire GP and later confined to its borders.
A normal MR imaging finding beyond the neonatal period but in the first year of life, should be controlled, especially when the typical clinical signs of kernicterus are present. Furthermore, MR imaging changes in the GP after 2 years of age may be subtle. When kernicterus is suspected clinically, thin slices in the diencephalon are recommended to avoid missing the pathology. Additionally, the mild neuroradiologic findings contrast with the severe movement disorder often observed in patients with chronic kernicterus.
Another significant finding was that the characteristic MR imaging pattern of kernicterus was frequently overlooked by pre-examiners, despite the presence of typical and severe clinical symptoms. In particular, when a neonatal MR image is not available, abnormalities on later MR images appear to be underrecognized.
An earlier diagnosis of kernicterus would have spared our patients the unnecessary diagnostic procedures, such as CSF analysis (patients 5 and 7), muscle biopsy (patient 6), and extensive genetic testing (patients 3, 4, 5, 6, 7). Moreover, MR imaging follow-up, which required sedation for these patients, would have been unnecessary if the diagnosis had been clear. This diagnosis would have alleviated stress for the patients and provided the families with earlier certainty regarding the diagnosis and prognosis of the disease.
A limitation of the study is that patients were identified individually during several years in 2 German university centers; thus, this study has no clear population basis. The emphasis is less on the prevalence of the problem but on the description of signal changes in the GP with time.
The patient group is small, reflecting the rarity of the disease currently. A larger patient group would be beneficial to validate our observations. Furthermore, our study focused on children up to 13 years of age. It would be interesting to investigate the further MR imaging course in adolescents and young adults with chronic kernicterus to understand whether the signal increase continues to decrease in the GP.
CONCLUSIONS
Despite the presence of typical MRI patterns, characteristic clinical history, and signs in all patients, the diagnosis of kernicterus was frequently overlooked. Especially, when a neonatal MR image with its typical signal increase on T1WI of the entire GP is not available, abnormalities on later MR images seem to be underrecognized. Furthermore, early infancy MR images may not reveal any pathology on T1WI or T2WI. The typical signal increase of the entire GP on T2WI becomes apparent only toward the end of the first year and beyond. Beyond the second year, the signal increase on T2WI is observed only at the borders of the GP.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received April 9, 2023.
- Accepted after revision June 25, 2023.
- © 2023 by American Journal of Neuroradiology