Abstract
BACKGROUND AND PURPOSE: The underlying mechanisms leading to altered cognitive, behavioral, and vision outcomes in children with prenatal opioid exposure are yet to be fully understood. Some studies suggest WM alterations in infants and children with prenatal opioid exposure; however, the time course of WM changes is unknown. We aimed to evaluate differences in diffusion tensor imaging MRI parameters in the brain between opioid exposed fetuses and normal controls.
MATERIALS AND METHODS: This is a pilot, prospective cohort study in which subjects in the third trimester of pregnancy underwent fetal DTI of the brain with 20 noncolinear diffusion directions and a b-value of 500 s/mm2 at 2.5-mm isotropic resolution.
RESULTS: The study included a total of 26 fetuses, 11 opioid-exposed (mean gestational age, 32.61 [SD, 2.35] weeks) and 15 unexposed controls (mean gestational age, 31.77 [SD, 1.68] weeks). After we adjusted for gestational age, fractional anisotropy values were significantly higher in opioid-exposed fetuses relative to controls in 8 WM tracts: the bilateral lemniscus (left: P = .017; right: P = .020), middle cerebellar peduncle (P = .027), left inferior cerebellar peduncle (P = .026), right sagittal stratum (P = .040), right fornix stria terminalis (P = .022), right inferior fronto-occipital fasciculus (P = .011), and the right uncinate fasciculus (P = .033). Significant alteration was also identified in other DTI indices involving a series of brain regions.
CONCLUSIONS: Our data demonstrate initial evidence of cerebral WM microstructural differences between opioid-exposed fetuses and unexposed controls. Further studies in larger patient populations will be needed to fully understand these findings.
ABBREVIATIONS:
- AD
- axial diffusivity
- FA
- fractional anisotropy
- GA
- gestational age
- MD
- mean diffusivity
- RD
- radial diffusivity
- SSFSE
- single-shot fast spin-echo
- SVR
- slice-to-volume registration
Opioid use during pregnancy remains a common problem in the United States, with 7% of pregnant women reporting the use of prescription opioid pain relievers during pregnancy and 0.8% of women having an opioid-related diagnosis at the time of delivery.1,2 Children with prenatal opioid exposure overall demonstrate lower educational achievement, compromised development, and higher rates of behavioral issues by school age.3⇓-5 However, the underlying neural mechanisms leading to these poor outcomes are still unknown. There is a growing body of literature describing neonatal brain differences in opioid-exposed infants, including lower regional brain volumes, increased WM injury, and alterations in functional connectivity.6⇓⇓⇓⇓-11 It is unknown whether these changes are present before birth.
Fetal MR imaging, which has become an accessible tool for the clinical evaluation of the developing brain, has the potential to answer some of these questions.12 Pilot studies using fetal MR imaging have described smaller brain sizes in fetuses with prenatal opioid exposure.13 DTI, a technique sensitive to WM abnormalities, has yielded growing evidence suggesting that opioid exposure may impact WM development in children as early as the neonatal period; however, WM microstructure in utero of opioid-exposed fetuses remains to be explored.14⇓-16 Fetal DTI has historically been challenging due to artifacts from excessive fetal motion. With recent advances in imaging data-processing and analysis, including slice-to-volume registration (SVR), fetal DTI has become more robust and reliable, making it possible to extend the study of WM microstructure into the prenatal period.17 This study aimed to assess WM integrity on the basis of in utero DTI from fetuses with opioid exposure during gestation.
MATERIALS AND METHODS
Study Design and Patients
This prospective study was approved by the institutional review board at each institution. Written informed consent was obtained from all study participants. Participants were recruited from the Cincinnati Children’s Hospital Medical Center (CCHMC) and the University of Arkansas for Medical Sciences (UAMS) from July 1, 2020, through December 31, 2021. Women in the third trimester of pregnancy with and without opioid use during the current pregnancy were recruited to undergo fetal MR imaging for investigational purposes only. Opioid and other substance use (or lack thereof) during the current pregnancy was determined by maternal self-report and maternal chart review. Substantial opioid use was defined as daily reported opioid use during most of the pregnancy to date, with most patients on a daily opioid-use disorder maintenance medication such as buprenorphine or methadone. Patients were recruited through a combination of flyers seeking volunteers in obstetrics and substance abuse clinics, e-mails to hospital employees seeking volunteers, and by connecting with previous research participants who agreed to be contacted for future research studies.
Eligibility for enrollment was determined by a study coordinator by telephone interview. Inclusion criteria included being at least 18 years of age, singleton pregnancy, and gestational age (GA) of at least 26 weeks. Exclusion criteria included an inability to supply the name of at least 1 additional person to contact if the participant could not be reached, a known genetic disorder, fetal abnormality identified on prenatal sonography, a nonviable fetus, contraindications to MR imaging, and the inability of the participant to enter the magnet bore due to body habitus. During this telephone interview, the study coordinator also informed potential participants that they would undergo a further interview regarding opioid exposure at the time of the fetal MR imaging appointment.
MR Imaging Acquisition
Fetal MR imaging examinations were performed using a 3T MR imaging system (Ingenia; Philips Healthcare) at CCHMC and a 3T system (Magnetom Prisma; Siemens) at UAMS. Both sites used a phased array abdominal imaging coil. Sedation was not used. Patients were placed in the left lateral decubitus position unless they reported feeling more comfortable in the supine position.
Examinations included localizer sequences followed by sagittal steady-state free precession images through the uterus with 5-mm-thick interleaved contiguous slices. T2 HASTE/single-shot fast spin-echo (SSFSE) images of the fetal brain were obtained in the axial, sagittal, and coronal planes with 2-mm-thick interleaved contiguous slices. Fetal DTI data were acquired in 20 noncolinear diffusion directions with a b-value of 500 s/mm2 and resolution of 2.5 mm isotropic.
MR Imaging Data-Processing and Analysis
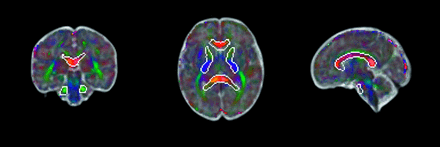
Orthogonally-acquired 2D stacks of T2 HASTE/ SSFSE images were motion-corrected and reconstructed into a single volumetric image with 1-mm3 isotropic resolution using the niftymic toolkit deployed in a docker image (https://hub.docker.com/r/renbem/niftymic), which additionally calculated an affine-registration matrix to a GA-matched template.18 Rigid-body transformation matrices describing the coregistration of the b=0 diffusion image to the reconstructed T2 HASTE image were calculated using the FMRIB Software Library (FSL; www.fmrib.ox.ac.uk/fsl), Version 6.0.4. DTI processing was performed using FSL as well. SVR was used to correct for excessive head motion and artifacts in the fetal DTI. Each acquired EPI section was individually aligned to a target estimation of the 3D fetal brain anatomy so that all data could be projected from the scanner coordinates to anatomic coordinates that are static relative to the fetal brain. DTI measures, including fractional anisotropy (FA) and mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD), respectively, were extracted from WM regions derived from a nonlinear registration of the Johns Hopkins University WM atlas (Mori et al,19 2005) into a GA-matched template space (Fig 1).20
Illustration of atlas-based WM regions outlined in coronal, axial, and sagittal directions. The examples of the brain regions include the following: 1) middle cerebellar peduncle; 2) genu, body, and splenium of the corpus callosum; and 3) bilateral anterior and posterior limbs of the internal capsule.
Statistical Analysis
Before being pooled for group-difference testing, multisite DTI data were harmonized using the ComBat approach, a statistical correction strategy that minimizes the intercenter effect resulting from scanner differences while preserving physiologic features.21 Group differences in FA, AD, MD, or RD in each atlas region were assessed using a FSL General Linear Model framework (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM) with opioid exposure status as a categoric predictor variable, GA as a continuous predictor variable (mean centered), and the DTI metrics as the dependent variables.
RESULTS
Description of Patient Sample
Forty-one mothers completed the MR imaging session: 28 from CCHMC (13 opioid-exposed, 15 controls) and 13 from UAMS (4 opioid-exposed, 9 controls). Of these scans, fetal DTI data of 15 mothers were excluded due to poor image quality (11 from CCHMC: 4 opioid-exposed, 7 controls; 4 from UAMS: 2 opioid-exposed, 2 controls). Thus, a total of 26 fetuses, including 11 opioid-exposed (GA: 32.61 [SD, 2.35] weeks; 9 from CCHMC, 2 from UAMS) and 15 unexposed controls (GA: 31.77 [SD, 1.68] weeks; P = .30; 8 from CCHMC, 7 from UAMS), were included in the final analyses. Other demographic characteristics are provided in the Table. No significant differences in fetal motion between the 2 groups were identified using analysis of average framewise displacement in millimeters (Wilcoxon rank-sum test, P = .96). All fetal brain MRIs were interpreted as having normal signal and morphology by the study radiologists.
Demographic information and additional substance exposure
Fetal DTI Findings
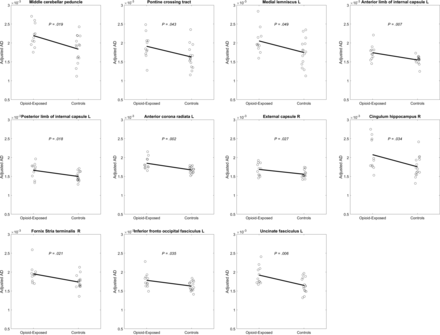
After adjusting for GA, FA values were significantly higher in opioid-exposed fetuses relative to controls in 8 WM regions (Fig 2). These significant regions included the bilateral lemniscus (left: P = .017; right: P = .020), the middle cerebellar peduncle (P = .027), the left inferior cerebellar peduncle (P = .026) and the right sagittal stratum (P = .040), the fornix (P = .022), the inferior fronto-occipital fasciculus (P = .011), and the uncinate fasciculus (P = .033). MD was significantly higher for exposed fetuses relative to controls in the left anterior limb of the internal capsule (P = .037) and the left uncinate fasciculus (P = .013) but significantly lower for exposed relative to controls in the left sagittal stratum (P = .035). AD was significantly higher for exposed fetuses relative to controls in 11 WM regions (Fig 3). These regions were the following: the middle cerebellar peduncle (P = .019), the pontine crossing tract (P = .043), the left medial lemniscus (P = .049), the anterior and posterior limbs of the internal capsule (P = .007, P = .018, respectively), the left anterior corona radiata (P = .002), the right external capsule (P = .027), the right cingulum hippocampus (P = .034), the right stria terminalis of the fornix (P = .021), the left inferior fronto-occipital fasciculus (P = .035), and the left uncinate fasciculus (P = .006). RD was significantly lower for exposed subjects relative to controls in the left sagittal stratum (P = .025).
Plots of FA, adjusted for age, by group in 8 deep WM structures showing opioid-exposed fetuses with higher FA compared with controls.
Plots of AD, adjusted for age, by group in 11 deep WM structures showing opioid-exposed fetuses with higher AD compared with controls.
DISCUSSION
This is a prospective study in which the use of ComBat harmonization allowed bi-institutional data use. There is a paucity of literature examining prenatal imaging of patients with in utero opioid exposure, and to the best of the authors’ knowledge, there are no previously published works examining DTI data in human fetuses with in utero opioid exposure. In this study, we observed statistically significantly increased FA values in opioid-exposed fetuses compared with healthy controls in 8 WM regions in the cerebrum, cerebellum, and brainstem after adjusting for GA. We also observed statistically significant alterations in AD in 11 different WM regions, MD in 3 regions, and RD in 1 region in the exposed fetuses relative to controls.
Alterations in DTI indices in children with prenatal exposure to opioids have been described in multiple age ranges and in association with various substances. There are studies describing alterations in neonates, infants, and school-aged children.16,22⇓-24 However, some of these changes are not consistently found in all studies in terms of the direction of alterations. For example, 1 study described decreased FA in prenatal methadone-exposed infants compared with controls.23 Another more recent study described significantly increased FA values in neonates (37- to 49-weeks’ postmenstrual age) with prenatal opioid exposure.25 The authors of this study also noted increased FA values in infants with prenatal exposure to cocaine and marijuana. Some of these previous works revealed region-specific direction changes in DTI parameters in the same cohort, reflecting the complexity of the underlying mechanisms of injury as a result of prenatal substance abuse on the developing brain.15,26
While our study potentially contributes one of the only descriptions of altered DTI parameters on fetal MR imaging in prenatal opioid exposure, the clinical significance of these results is preliminary in nature. Previous work examining the relationship between DTI and histology has demonstrated that, in general, abnormally lower FA values and higher MD, AD, and/or RD values are often interpreted as damage to the myelin sheath and axonal membrane. However, higher FA and lower MD, AD, and/or RD values can also be attributed to extracellular space compression, cytotoxic edema, or inflammation.27,28 In the present study, we identified multiple WM regions with abnormally higher FA, driven mainly by the increase of AD, a result that seems to be in line with the latter scenario. Increased expression of inflammatory genes has also been demonstrated in infants with prenatal opioid exposure compared with unexposed controls, making the underlying inflammatory processes a potential explanation for our findings.29 However, contrary to the direction of changes observed in these studies, 1 mouse model study demonstrating increased serum inflammatory biomarkers described reduced WM FA on ex vivo DTI of the brain with decreased axial diffusivity.30 Also, the interpretation of underlying neuropathology and its potential association with substance abuse during fetal life is complicated by the rapid fetal brain development and maturation process. Furthermore, our statistical analysis of group differences assumed a linear relationship in the developmental trajectory for DTI, which may not be accurate.
The current literature in fetal DTI is evolving, and while some studies have reported a linear or at least monotonic relationship between DTI and age, other studies have revealed a more complicated temporal process. For example, as reported in a study by Zanin et al,31 the potential relationship between DTI and age may vary as determined by different phases during fetal brain maturation. While no significant age correlation was found for DTI in our data, this finding could be due to the limited sample size in our study, which did not allow further exploration of this relation. Therefore, future studies with larger sample sizes are critical in elucidating underlying injury mechanisms along with spatiotemporal progression in the fetal brain with opioid exposure.32
Our study has limitations. One of the major limitations of this study is the relatively small sample size. The issue was partially addressed through bi-institutional collaboration and the use of ComBat harmonization. However, additional studies in larger patient populations will be needed to further understand and validate these results. Another limitation commonly encountered in fetal imaging is motion artifacts, which are one of main challenges faced in fetal DTI performance. Despite using SVR to improve image quality, multiple patients had to be excluded for excessive fetal motion in this study. Super resolution reconstruction methods have been shown to improve the image and data quality by scanning in multiple orthogonal planes and show promise for future studies though they come at the cost of increased imaging time.33 Finally, one of the main limitations of this study relates to the opioid-exposed patient population and the potential for confounding variables. In the opioid-exposed cohort, 54.5% (6/11) of mothers had reported prenatal exposure to nicotine, other illicit drugs, or both, which can affect brain development. Also, fetal brain development may have been impacted by a range of additional factors such as maternal nutrition, stress, and other environmental factors.
CONCLUSONS
This study demonstrates the feasibility of the application of fetal DTI, a highly challenging technique, to quantitatively assess WM integrity in opioid-exposed fetuses. Our multisite data show widespread WM regions with significant DTI abnormalities in the patients, which we hypothesize to be due to prenatal opioid exposure causing impairment during the complex series of neurogenic events (neurogenesis, neuronal migration, synapsis, axonal growth, myelination) in the fetal development and maturation process. Early detection of such abnormalities as well as their progression will provide critical data to inform prenatal counseling, treatment, and intervention strategies with the ultimate aim of optimizing long-term outcomes.
Footnotes
This work was supported by the Schubert Research Clinic Clinical Research Feasibility Fund, Cincinnati Children’s Radiology Pilot Fund, and grants CCHMC R34-DA050268 and R34-DA050261 from Phase I of the HEALthy Brain and Child Development Study.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
J.A. Dudley and U.D. Nagaraj contributed equally to this work.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 13, 2023.
- Accepted after revision June 25, 2023.
- © 2023 by American Journal of Neuroradiology