Abstract
SUMMARY: The management of acute ischemic stroke has undergone a paradigm shift in the past decade. This has been spearheaded by the emergence of endovascular thrombectomy, along with advances in medical therapy, imaging, and other facets of stroke care. Herein, we present an updated review of the various stroke trials that have impacted and continue to transform stroke management. It is critical for the radiologist to stay abreast of the ongoing developments to provide meaningful input and remain a useful part of the stroke team.
ABBREVIATIONS:
- AHA/ASA
- American Heart Association/American Stroke Association
- ACS
- anterior circulation stroke
- AIS
- acute ischemic stroke
- COR
- class of recommendation
- eTICI
- expanded TICI
- EVT
- endovascular thrombectomy
- ICAD
- intracranial atherosclerotic disease
- ICH
- intracranial hemorrhage
- LOE
- level of evidence
- LVO
- large-vessel occlusion
- mTICI
- modified TICI
- PCS
- posterior circulation strokes
- RCT
- randomized controlled trial
- SMM
- standard medical management
- TNK
- tenecteplase
Every year, around 800,000 individuals experience new or recurrent strokes, with most of these being new cases. Approximately 87% are ischemic, 10% reflect intracranial hemorrhage (ICH), and 3% are SAH. Despite a general decrease in stroke incidence during the past 30 years, it is projected that by 2030, an additional 3.4 million US adults will have had a stroke.1
The management of acute ischemic stroke (AIS) has undergone a remarkable transformation in the past decade, largely led by endovascular thrombectomy (EVT), with contributions through improvements in thrombectomy devices, medical management, and stroke workflows. Recent trials have also demonstrated improved outcomes with EVT in posterior circulation strokes (PCS) and larger strokes, which will continue to impact stroke care in the future. Additionally, various aspects of stroke therapy are currently being studied, including the role of EVT in distal occlusions and clinically mild strokes with large-vessel occlusion (LVO). It is crucial for radiologists and the medical community to stay informed about these developments to provide meaningful information that positively impacts patient outcomes. To this end, a review of recent studies related to AIS is presented.
Before proceeding, it is important for the reader to understand 3 commonly used terms in stroke care: the mRS, the NIHSS, and the modified TICI (mTICI) scale. The mRS is a 7-point scale that ranges from 0 (no symptoms) to 6 (death) and covers the entire range of functional outcomes in stroke. It is easy to administer, correlates with measures of stroke, and is useful in evaluating the efficacy of acute stroke therapies. A single-point change in the mRS score is considered clinically relevant.2 In all trials conducted to assess stroke outcomes, at least one of the primary end points is typically the mRS, due to its high validity and reliability as well as its requirement for a smaller sample size compared with other measures of stroke outcomes.3
The NIHSS is a 15-item neurologic examination scale to assess stroke severity and changes in clinical status. The score ranges from 0 to 42, with higher scores indicating greater stroke severity. A recent meta-analysis noted that an NIHSS score of ≥10 is 73% sensitive and 74% specific for predicting underlying LVO.4 The 2019 update to the 2018 American Heart Association/American Stroke Association (AHA/ASA) guidelines also recommend the use of a stroke scale, preferably the NIHSS to assess stroke severity.5
Finally, the mTICI was derived from the original TICI grading in 2013 and measures the degree of reperfusion.6 The score ranges from 0 to 3 with grade 0 indicating no reperfusion, grade 1 indicating limited distal filling past the initial occlusion, grade 2 indicating further reperfusion with subdivisions based on the amount of reperfused MCA territory (2a: <50%; 2b: >50%; 2c: 90%–99%), and grade 3 indicating complete reperfusion. The current AHA/ASA guidelines recommend a score of ≥2b as the angiographic goal of EVT.5 More recently, an expanded TICI (eTICI) scale has also been proposed, which encompasses a 7-point score, with eTICI grade 0 implying no reperfusion and grade 1 implying thrombus reduction without distal reperfusion. eTICI 2 is further subdivided to define the extent of reperfusion more precisely (2a: 1%–49%; 2b50: 50%–66%; 2b67: 67%–89%; 2c: 90%–99%), while eTICI 3 implies complete reperfusion, similar to TICI 3.7 The authors noted that despite adjacent categories of reperfusion in eTICI 2, the outcomes were significantly different, and they argued that eTICI 2b67 could be considered the ideal threshold for defining successful reperfusion.
Thrombolytics
IV-tPA was first approved by the FDA for the treatment of AIS within 3 hours of symptom onset in 1995, based on the results of the for National Institute of Neurological Disorders and Stroke tPA (NINDS tPA) trial.8 In 2008, the treatment window for IV tPA was expanded to 4.5 hours, after the European Cooperative Acute Stroke Study III demonstrated sustained treatment benefits.9 More recently, the European Cooperative Acute Stroke Study (ECASS) IV and Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND) trials evaluated IV tPA efficacy between the 4.5- and 9-hour window in patients who were not EVT eligible and had a perfusion-to-diffusion mismatch ratio of ≥1.2. Although ECASS IV did not show significantly improved 90-day outcomes, EXTEND demonstrated better functional independence when adjusted for age and stroke severity. Both studies were, however, terminated early. Enrollment in ECASS IV declined post publication of extended window EVT trials, and EXTEND was stopped because the results from the Efficacy and Safety of the MRI-Based Thrombolysis in Wake-Up Stroke (WAKE-UP) trial led to loss of equipoise.10,11
The safety and efficacy of IV tPA in AIS of unknown onset, which accounted for 14%–27% of strokes, was evaluated in the WAKE-UP and A Study of Intravenous Thrombolysis With Alteplase in MRI-Selected Patients (MR WITNESS) trials.12,13 Of note, MR WITNESS was primarily designed to assess the safety rather than the efficacy of IV tPA administration. WAKE-UP used DWI-FLAIR mismatch criteria (ischemic lesion visible on DWI without corresponding FLAIR signal change), while MR WITNESS further quantified the FLAIR signal change (FLAIR signal intensity in ischemic region/contralateral normal brain ≤1.15) as a criterion for IV tPA administration. Even though WAKE-UP was terminated early due to lack of funding, analysis of the 503 enrolled patients revealed that 53.5% of the IV tPA group achieved a 90-day mRS of 0–1 compared with 41.8% in the placebo group (P = .02).
Tenecteplase (TNK) is a bioengineered form of alteplase with higher fibrin selectivity and a longer half-life, allowing administration as a single bolus dose, unlike alteplase, which is given initially as a bolus (10% dose) followed by slow infusion (90% dose) for 1 hour. The Tenecteplase versus Alteplase before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK) showed that TNK administration before EVT resulted in improved revascularization and 90-day outcomes, compared with alteplase.14,15 More recently, Tenecteplase versus Alteplase in Acute Ischaemic Cerebrovascular Events (TRACE-2) (1430 patients) and Alteplase compared to Tenecteplase in Patients With Acute Ischemic Stroke (AcT) (1600 patients) showed TNK to be noninferior to alteplase in patients presenting within 4.5 hours of AIS.16 TNK is, however, currently not FDA-approved for AIS, though this may change in the future, given the accumulating evidence.
Thrombolytics, however, have limitations such as a narrow treatment window and modest recanalization rates for LVO, prompting a search for alternate methods to achieve timely recanalization.
EVT in Anterior Circulation Stroke
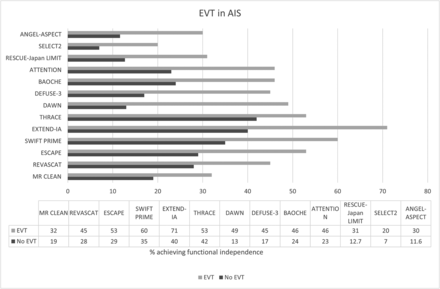
In 2015, five randomized controlled trials (RCTs) showed the efficacy of EVT over standard medical management (SMM) in patients with anterior circulation stroke (ACS) with proximal LVO. The Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) found that 32% of patients treated with EVT had better outcomes (90-day mRS 0–2) compared with 19% in the SMM group.17 Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) trial ended early after MR CLEAN study results were released, with the interim analysis showing higher functional independence rates with EVT (53%) compared with SMM (29%). The ESCAPE trial focused on efficient workflow, emphasized the use of CTA over MR imaging, and achieved a median NCCT-to-reperfusion time of 84 minutes.18 Similar positive results were also noted with the Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment (SWIFT PRIME), Extending the Time for Thrombolysis in Emergency Neurological Deficits Intra-Arterial (EXTEND-IA), Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours (REVASCAT), and the Trial and Cost Effectiveness Evaluation of Intra-Arterial Thrombectomy in Acute Ischemic Stroke (THRACE studies) (Figure).19⇓⇓-22
Barplot showing the differences in functional outcomes with (gray) and without EVT (black) in select trials. The first 3 trials evaluated EVT with large infarct, and BAOCHE and ATTENTION assessed EVT in PCS, while the rest assessed EVT in ACS.
A meta-analysis of the 5 major EVT trials, conducted in 2016 by the HERMES collaboration, found that the effectiveness of EVT declined with each passing hour and concluded that treatment within the first 7 hours is likely to produce the best results.23 The number needed to treat was 2.6.24 Subsequently, the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trials were undertaken and demonstrated the effectiveness of EVT in delayed time windows (up to 24 hours).25,26 The DAWN trial showed increased functional independence in patients in the 6- to 24-hour window who had a clinical-imaging mismatch. The DEFUSE 3 trial, on the other hand, showed improved outcomes with EVT for patients presenting in the 6- to 16-hour time window who met certain imaging criteria (ACS with LVO involving the ICA or proximal MCA, core infarct of <70 mL, and ratio of ischemic tissue to infarct of >1.8), regardless of clinical-imaging mismatch. Of note, about 40% of patients in DEFUSE 3 would not have met the clinical-imaging mismatch criteria of the DAWN trial. The DEFUSE 3 trial was terminated early after an early interim analysis established EVT superiority.
More recently, a few trials have evaluated the role of combined IV tPA and EVT compared with EVT alone. The Direct Intra-Arterial Thrombectomy in Order to Revascularize AIS Patients With Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals (DIRECT-MT) and Effect of Endovascular Treatment Alone Versus Intravenous Alteplase Plus Endovascular Treatment on Functional Independence in Patients with Acute Ischemic Stroke (DEVT) trials (China) showed noninferiority of EVT compared with combined therapy.27,28 However, the Randomized study of endovascular therapy with versus without intravenous tissue plasminogen activator in acute stroke with ICA and M1 occlusion (SKIP) trial (Japan) failed to show noninferiority, while the Solitaire With the Intention for Thrombectomy plus Intravenous tPA Versus DIRECT Solitaire Stent-Retriever Thrombectomy in Acute Anterior Circulation Stroke (SWIFT-DIRECT) trial (Europe and Canada) noted that EVT alone was not noninferior and resulted in reduced reperfusion rates compared with combined therapy.29,30 The Multicenter MR CLEAN-NO IV trial (Europe) also noted that EVT was neither superior nor noninferior to combined therapy.31 More recently, A Randomized Controlled Trial of DIRECT Endovascular Clot Retrieval versus Standard Bridging Thrombolysis With Endovascular Clot Retrieval (DIRECT-SAFE) again did not show noninferiority of EVT. Most interesting, the authors noted better outcomes in Asian patients with combined therapy.32 Currently, Endovascular Treatment With versus Without Intravenous rhTNK-tPA in Stroke (BRIDGE-TNK, NCT04733742) is currently evaluating a combination of TNK and EVT compared with EVT alone.
EVT in Large Core Infarcts
In the past, most trials excluded patients with ASPECTS of < 5 or a core infarct volume of >70 mL. These patients, however, have poor outcomes. Recently, the RCT of Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan Large IscheMIc core Trial (RESCUE-Japan LIMIT), a prospective RCT that enrolled patients with ASPECTS between 3 and 5, showed improved functional outcomes (90-day mRS 0–3) in patients who additionally received EVT compared with SMM.33 Although the EVT group had a higher incidence of ICH, the incidence of symptomatic ICH was not significantly different between groups. A secondary analysis suggested that improved functional outcomes were mainly seen in patients with ASPECTS of 4 or 5, whereas those with ASPECTS of ≤3 did not have significantly improved outcomes.34 Earlier in 2023, two additional trials, A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT2) and Endovascular Therapy in Acute Anterior Circulation Large VeSsel Occlusive Patients with a largE infarCT core (ANGEL-ASPECT) evaluated patients with ASPECTS between 3 and 5 and large-core infarct (ANGEL-ASPECT, 70–100 mL, and SELECT2, ≥ 50 mL). Both trials were stopped early due to overwhelmingly improved outcomes with EVT.35,36 Currently, at least 3 more trials are evaluating EVT in large infarcts, including the Large Stroke Therapy Evaluation (LASTE), Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window (TENSION), and Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke (TESLA).37
Tandem Occlusions
Tandem lesions, defined as anterior circulation LVOs with concurrent high-grade stenosis or occlusion of the ipsilateral ICA secondary to atherosclerosis or vascular dissection, may be seen in up to 10%–20% of patients with AIS. Their optimal management remains unclear. A subgroup analysis of the Safety and Efficacy of Nerinetide (NA-1) in Subjects Undergoing Endovascular Thrombectomy for Stroke (ESCAPE-NA1) data showed that a concurrent tandem lesion did not lower the odds of a good functional outcome, regardless of stent placement.38 However, because the trial enrolled only patients with moderate collaterals and smaller infarcts, the confounding effect of these variables remains unclear. A recent meta-analysis reviewing the effect of acute carotid stent placement in patients undergoing EVT noted that stent placement was associated with favorable outcomes without increased mortality or ICH.39 Ongoing RCTs, the Endovascular Acute Stroke Intervention–Tandem OCclusion Trial (EASI-TOC) (NCT04261478), Proximal Internal Carotid Artery Acute Stroke Secondary to Tandem or Local Occlusion Thrombectomy Trial (PICASSO, NCT05611242), and Thrombectomy In TANdem Occlusion (TITAN, NCT03978988), will prospectively evaluate the role of carotid stent placement in AIS with tandem lesions.38
EVT in PCS
Posterior circulation LVOs may account for 7%–12% of all intracranial LVOs in AIS, and up to 80% of patients with basilar artery occlusion presenting with moderate-to-severe deficits die or have severe disability despite SMM.40⇓-42 The most frequent causes of posterior circulation LVOs in the MR CLEAN registry were large-artery atherosclerosis and cardioembolism.43 Four recent RCTs evaluated the role of EVT in PCS. The Acute Basilar Artery Occlusion: Endovascular Interventions vs Standard Medical Treatment (BEST) trial was discontinued due to poor recruitment and high crossover rates between the treatment arms.44 The Basilar Artery International Cooperation Study (BASICS) did not demonstrate any significant additional benefit with EVT.45 However, the study had nonconsecutive enrollment, and a third of eligible patients were treated outside the trial, most of whom received EVT, which may have biased the study conclusions.
More recently, the Basilar Artery Occlusion Chinese Endovascular (BAOCHE) and Endovascular Treatment For Acute Basilar Artery Occlusion (ATTENTION) trials, both conducted in Chinese patients, showed improved outcomes with EVT in PCS presenting between 6 and 24 hours and <12 hours of last known well, respectively.41,46 Of note, both trials used posterior circulation ASPECTS as one of the exclusion criteria, with BAOCHE excluding patients with a score of <6, and ATTENTION excluding patients with a score of <6 if younger than 80 years of age and a score of <8 if older than 80 years of age.
Thrombectomy Devices
The mechanical embolus removal in cerebral ischemia (Merci) device (Stryker) was the first successful clot-retrieval device, achieving recanalization in 48% of patients.47 This was followed by the Penumbra system (Penumbra), which achieved Thrombolysis in Myocardial Infarction scores of 2–3 in 81.6% of patients.48 However, early studies raised concerns about the efficacy of EVT, failing to show any additional benefit compared with SMM. Importantly, these studies did not require LVO confirmation for enrollment into the treatment arm.49,50
It was only after the publication of MR CLEAN and subsequent trials in 2015 that there was renewed interest in EVT.17,18,20⇓-22 Most of these trials used second-generation thrombectomy devices known as stent retrievers.
Stent retriever and clot aspiration are the 2 most used EVT techniques, with continued improvements leading to reperfusion rates exceeding 90% in LVO thrombectomy.51 Stent retrievers are inserted within the thrombus and re-establish blood flow once expanded, while simultaneously binding the thrombus. Subsequent stent retrieval extracts the clot. Aspiration devices, on the other hand, connect to an external aspiration pump that creates a negative suction to aspirate the clot. The Contact Aspiration vs Stent Retriever for Successful Revascularization (ASTER) trial, a multicenter, randomized blinded-end-point superiority trial conducted in France failed to show superiority of first-line contact aspiration compared with a first-line stent retriever in AIS.52 The COMPASS Trial: a Direct Aspiration First Pass Technique (COMPASS) trial, on the other hand, was designed as a noninferiority trial and conducted in a multicenter setting in North America. The study compared the 2 techniques and showed that contact aspiration was noninferior to stent retrievers.53 Currently, the choice of technique is primarily based on user preference and expertise.
AIS Secondary to Intracranial Atherosclerotic Disease
Intracranial atherosclerotic disease (ICAD) accounts for about 10%–15% of AIS cases in the West but has higher prevalence in Asia where it may account for up to 46.6% of AIS cases.54 These patients have an especially higher risk of recurrent stroke and often have acute vessel re-occlusion despite repeat recanalization during EVT.51,54 The Wingspan stent (Stryker) was the first self-expanding stent designed specifically for ICAD and is the only FDA-approved stent for symptomatic ICAD.55 A few trials have evaluated the role of stent placement versus aggressive medical therapy in preventing recurrent strokes in this cohort. The Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial was terminated early due to a significantly higher complication rate in the stented group.56 Similarly, the Vitesse Intracranial Stent Study for Ischemic Therapy (VISSIT) trial was also terminated early after an interim analysis showed increased stroke risk with stent placement.57 More recently, the China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) trial again showed no additional benefit of stent placement over medical management.54 However, 2 recent postmarketing surveillance studies (Post Market Surveillance Study of the Wingspan Stent System [WEAVE] trial and the Post Market Surveillance of Percutaneous Transluminal Angioplasty and Wingspan Stenting for Intracranial Atheroslerotic Disease [WICAD] study) demonstrated an excellent safety profile when used by experienced interventionalists following on-label guidelines. Functional independence (mRS 0–2) was achieved in 88.9% of patients in the WICAD group with a mortality rate of 3%.58,59
Role of Imaging in AIS
The current AHA/ASA guidelines recommend that all patients suspected of having AIS undergo brain imaging on first arrival (class of recommendation [COR] I; level of evidence [LOE] A). Both NCCT (COR-I; LOE-A) and MR imaging (COR-I; LOE-B) are effective in excluding ICH before IV tPA.5 Of note, a post hoc analysis of the THRACE data using 401 patients (299 MR imaging and 102 CT) did not note any differences in clinical outcomes despite the slightly longer duration of the MR imaging scans (median MR imaging duration, 13 minutes; CT, 9 minutes).60
For patients with AIS presenting between 6 and 24 hours, the guidelines recommend CTA with CT perfusion or MRA with DWI with or without MR perfusion for selecting EVT candidates (COR-I; LOE-A). However, in patients presenting <6 hours from last known well and having an ASPECTS of >6, EVT eligibility may be determined on the basis of NCCT and CTA/MR imaging and MRA without additional perfusion studies (COR-I; LOE-B). In patients with AIS who awake with stroke symptoms or have an unclear time of onset of >4.5 hours from last known well or a baseline state, MR imaging to detect DWI-FLAIR mismatch may be useful in selecting IV tPA–eligible patients (COR-IIa; LOE-B).
The guidelines also recommend noninvasive intracranial vascular imaging for patients who otherwise meet the EVT criteria or when LVO is suspected (COR-I; LOE-A). Extracranial carotid and vertebral artery imaging may be reasonable to provide information on eligibility and treatment-planning (COR-IIb; LOE-C). Incorporating the collateral status into decision-making for potential EVT is also considered reasonable (COR-IIb; LOE-C).
Several post hoc analyses of published RCTs and larger patient registries have evaluated the role of imaging markers in the assessment of stroke. Boodt et al,61 in an analysis of 1429 consecutive patients from the MR CLEAN registry, noted that noncardioembolic strokes were associated with the presence of the hyperdense artery sign (OR = 2.2; 95% CI, 1.6–3.0) and a more proximal thrombus location (common OR = 0.2; 95% CI, 0.2–0.3), findings based on univariable analysis. Additionally, thrombus characteristics in strokes with undetermined etiology were similar to those of cardioembolic strokes, suggesting that most cryptogenic strokes may be cardioembolic. A secondary analysis of the DIRECT-MT trial noted that the hyperdense artery sign at baseline indicated improved outcomes with the addition of IV tPA to EVT, while its absence correlated with worse outcomes.62
Both the hyperdense artery sign and increased susceptibility of the thrombus on T2* images (positive susceptibility vessel sign) are secondary to an increased amount of red blood cells, which can favorably interfere with stent retriever struts during EVT.61,63 A subgroup analysis of the THRACE data noted that smaller DWI volumes, the presence of a positive susceptibility vessel sign, and a short susceptibility vessel sign length were associated with excellent outcomes (90-day mRS ≤1) with IV tPA alone.64
A post hoc analysis of the DEFUSE 3 data noted that nearly half of the penumbral tissue with a time-to-maximum of >6 seconds may remain viable in untreated patients at 24 hours, while about 74% of the penumbral tissue with time-to-maximum of >10 seconds may remain viable after TICI 3 recanalization. Similar effects on penumbral tissue were, however, not seen with incomplete recanalization (TICI 0–2b).65 Another secondary analysis of the DEFUSE 3 data noted that patients with favorable collaterals had smaller 24-hour infarct volumes than initially predicted, suggesting that collateral status may impact final infarct volumes.66
In terms of functional outcomes, a post hoc analysis of the ESCAPE-NA1 using 1026 patients noted that infarction confined to gray matter, sparing of the corticospinal tract, and scattered infarct structure were highly predictive of good 90-day outcome.67 Hemorrhagic transformation, regardless of severity, is associated with worse functional outcomes, though the effect appears more pronounced with hemorrhage of >30% of the infarct volume.68 A substudy of THRACE data noted that pretreatment infarct volume is an independent predictor of functional outcome. The efficacy of EVT decreases with increasing infarct volume, with the number of patients needed to treat to achieve functional independence increasing from 10 patients for a volume of 80 mL to 15 patients for a volume of 135 mL.69 The Online Supplemental Data outline imaging-based inclusion and exclusion criteria of select stroke trials based on thrombolytics and EVT, respectively.
Stroke Workflow and Perioperative Management
Because the most critical component in AIS is timely re-establishment of perfusion, several studies have evaluated the impact of different workflows and perioperative management on overall outcomes.
The Direct Transfer to an Endovascular Center Compared to Transfer to the Closest Stroke Center in Acute Stroke Patients With Suspected Large Vessel Occlusion (RACECAT) study, which evaluated differences in outcomes in nonurban areas between patients who were transferred directly to an EVT-capable center compared with patients who were initially transferred to a local stroke center (capable of imaging, IV tPA, but not EVT), followed by transfer to an EVT-capable center if LVO was confirmed, was halted for futility after the second interim analysis showed no differences in outcomes.70 The best approach, therefore, may be based on the availability of local resources and achievable workflow metrics, and a “drip and ship” approach may still be acceptable, especially in remote settings.
Because multiple prior studies have demonstrated improved patient outcomes with earlier treatment initiation, considerable effort has been made to reduce door-to-needle times.23,71 More recent studies have demonstrated that door-to-needle times of <60 minutes are achievable in most patients.72 Another recent study noted that for patients with ACS undergoing EVT, the addition of 100% oxygen through a face mask led to significantly reduced infarct volumes at 24–48 hours (median, 20.1 versus 37.7 mL; P < .01) and improved 90-day mRS.73 Two recent trials evaluated the safety and efficacy of intensive blood pressure control after EVT. The Blood Pressure Target in Acute Stroke to Reduce Hemorrhage After Endovascular Therapy (BP-TARGET) trial did not find a difference in radiographic intraparenchymal hemorrhage, while the Second ENhanced Control of Hypertension ANd Thrombectomy strokE stuDy (ENCHANTED2/MT) trial was stopped early after outcome data revealed that more intensive blood pressure control was associated with poor functional outcomes and early neurologic deterioration.74,75
In terms of sedation during EVT, the General Or Local Anaestesia in Intra Arterial Therapy (GOLIATH), Anesthesia during Stroke (AnStroke), Sedation vs Intubation for Endovascular Stroke TreAtment Trial (SIESTA), and General Anesthesia vs Sedation During Intra-arterial Treatment for Stroke (GASS) studies showed that both conscious sedation and general anesthesia were equally effective in terms of neurologic improvement and 90-day functional outcomes.76⇓⇓-79 However, 3 of these are single-center studies and had smaller sample sizes.76,78,79 A recent meta-analysis of 7 RCTs with a total of 980 patients (487 general anesthesia, 493 non-general anesthesia) showed that general anesthesia was associated with higher rates of recanalization, resulting in 8.4% absolute improvement in the rate of good functional outcome.80 The current AHA/ASA guidelines recommend technique selection based on individualized assessment, clinical characteristics, and technical performance of the procedure.5
Future Directions
Despite the advances, multiple aspects of stroke treatment should be further addressed. As discussed earlier, ongoing trials will further refine the role of EVT in large infarcts. An LVO may be seen in up to 28% of patients with AIS and an NIHSS score of ≤4, and the best LVO treatment strategy in clinically mild stroke (NIHSS score of ≤5) is unclear. The Endovascular Therapy for Low NIHSS Ischemic Strokes (ENDOLOW, NCT04167527) and Minor Stroke Therapy Evaluation (MOSTE, NCT03796468) trials are currently underway to study this issue.
Similarly, treatment strategies in distal medium-vessel occlusions remain unclear. Medium vessels (defined as A2/A3 ACA, M2/M3 MCA, and P2/P3 PCA vessels) were generally excluded from prior EVT trials but can result in substantial neurologic deficits. Meta-analysis of the HERMES group data noted that patients with M2 MCA occlusions would benefit from EVT (adjusted OR = 2.39; 95% CI 1.08–5.28; P = .03), with the number needed to treat for 1 patient to achieve functional independence being 5.4.81 Rescue Thrombolysis for mEdium veSsel oCclUsion (RESCUE-TNK, NCT05657470) is currently evaluating the role of intra-arterial TNK in both primary (de novo) and secondary (to EVT) medium-vessel occlusions. The effect of EVT in distal occlusions is also currently being evaluated in prospective studies: Distal Ischemic Stroke Treatment with Adjustable Low-profile Stentriever (DISTALS, NCT05152524) and Evaluation of Mechanical Thrombectomy in Acute Ischemic Stroke Related to a Distal Arterial Occlusion (DISCOUNT) (NCT05030142).
Patients who achieve reperfusion during the first attempt of EVT have improved functional outcomes, known as the first-pass effect. A recent metanalysis comprising 2747 patients noted that patients with a first-pass effect had better outcomes and lower mortality, especially if they achieved mTICI 3 recanalization.82 This finding has increasingly led to comparison of catheter performance in terms of achieving a first-pass effect mTICI≥ 2b.51 The use of balloon-guided catheters during EVT is also being explored to reduce the chances of clot fragmentation and distal embolization.83
In terms of thrombolytic therapies, the Norwegian Tenecteplase Stroke Trial 2 (NOR-TEST 2, NCT03854500) is evaluating TNK within 4.5 hours of stroke onset. The Tenecteplase in Stroke Patients Between 4.5 and 24 Hours (TIMELESS, NCT03785678) study is a Phase III trial evaluating TNK in the late-therapy time window, while Randomization to Extend Stroke Intravenous ThromboLysis In Evolving Non-Large Vessel Occlusion With TNK [RESILIENT EXTEND-IV, NCT05199662]) is assessing TNK in patients with AIS without LVO within the 4.5- to 12-hour window. The Extending the Time Window for Tenecteplase by Effective Reperfusion in Patients With Large Vessel Occlusion (ETERNAL-LVO, NCT04454788) is similarly evaluating the role of TNK in EVT-eligible patients presenting within 24 hours, while the Extending the Time Window for Tenecteplase by Recanalization of Basilar Artery Occlusion in Posterior Circulation Stroke (POST-ETERNAL, NCT05105633) trial is evaluating the same for PCS.
Finally, even though no neuroprotective agents are currently approved by the US FDA, there is increasing focus on both pharmacologic and nonpharmacologic therapies, which reduce excitotoxicity, oxidative stress, inflammation, or cellular apoptosis in AIS. Initial studies using uric acid and nerinetide have shown improved neuroprotection, especially in patients undergoing EVT. Multiple prospective trials are ongoing to further identify and refine the role of neuroprotective agents in AIS.84
CONCLUSIONS
The past decade has seen a paradigm shift in the management of AIS. These trends will likely continue, and ongoing trials are expected to further refine AIS care, with likely a much more nuanced and granular approach in individual cases. The radiologist will need to stay abreast of these developments to provide a meaningful contribution to patient care and remain an important part of the care team.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- Received March 5, 2023.
- Accepted after revision April 3, 2023.
- © 2023 by American Journal of Neuroradiology