Abstract
SUMMARY: Pedicled locoregional submandibular gland reconstruction flaps are increasingly used in oncologic head and neck surgery and have unique imaging characteristics that can mimic locally recurrent tumor. In this clinical report, 23 posttreatment imaging studies were evaluated in 19 patients who had undergone submandibular gland flap reconstructions after resection of a primary head and neck tumor. Submandibular gland flaps were most commonly mobilized into the parapharyngeal space or parotid bed, with others located inferior to the mandibular body and within marginal mandibulectomy defects. The original shape of the gland was typically not preserved. Identifying the submandibular gland hilum, vascular pedicle, glandular texture, and absence of submandibular gland in the orthotopic location was most useful in recognizing a flap. The interpreting radiologist must be familiar with the unique submandibular gland flap imaging characteristics to accurately differentiate normal postoperative appearance and recurrent tumor.
ABBREVIATIONS:
- SCC
- squamous cell carcinoma
- SMG
- submandibular gland
Surgical flaps are commonly encountered during surveillance imaging following oncologic resection in the head and neck, particularly as advancements in microvascular surgical techniques have facilitated the widespread use of free flaps. The resultant complex postoperative anatomy can make imaging interpretation challenging. To aid the interpreting radiologist, multiple studies have characterized the typical postoperative imaging appearance of various fasciocutaneous and myocutaneous flap reconstructions in the head and neck.1⇓-3 To our knowledge, existing studies have not characterized the imaging appearance of pedicled locoregional glandulofascial flaps involving the submandibular gland (SMG).
First described by Mozolewski et al4 for laryngeal reconstruction, the SMG flap has since been described for the reconstruction of small-to-medium defects that cannot be closed primarily yet may not necessitate the additional risk and surgical complexity of free flaps.5 From a surgical perspective, the benefits of a pedicled regional SMG flap include an abundant blood supply from the facial artery, the option to include surrounding adipose tissue for bulk, and a relatively long arc of rotation, thereby allowing mobilization to sites as far as the infratemporal fossa or parotid bed.6,7 Furthermore, in instances when level 1 nodes will be dissected, no additional incision or secondary surgical defect is required, unlike the temporalis myofascial or pectoralis flaps.5⇓-7 In comparison with myocutaneous flaps, an SMG flap retains greater bulk across time, therefore obviating surgical overestimation of tissue volume necessary to reconstruct a defect.6 This feature may more accurately restore a desired facial contour after parotidectomy or preserve mucosal volume of an oropharyngeal defect in which the swallowing function could be eventually impaired by flap atrophy. Of note, SMG transfer to the submental space with the intent of avoiding the high-dose radiation field to avoid xerostomia is a distinct entity and not included herein.8 A key difference between these entities is that SMG transfer is performed on the SMG contralateral to the site of disease, whereas SMG flap reconstruction is performed ipsilesionally.
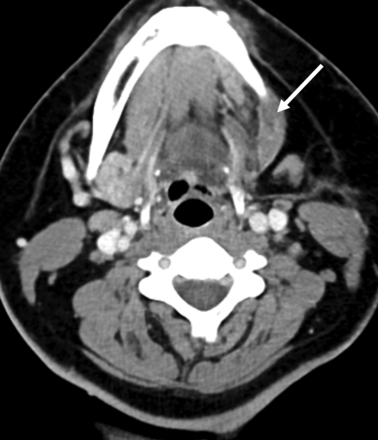
As previously described in the literature, the postlaryngectomy imaging appearance of a mobilized thyroid gland can simulate recurrent tumor.9 Likewise, the postoperative appearance of an SMG flap reconstruction may consist of hyperattenuating, nodular tissue in the primary resection site, whereby an interpreting radiologist who is unfamiliar with this technique may easily mischaracterize normal flap reconstruction for recurrent tumor (Fig 1). The purpose of this study was to characterize the normal CT and MR imaging postoperative appearance of SMG flaps to avoid this pitfall in the posttreatment setting.
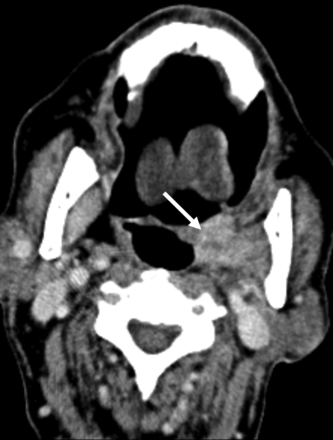
SMG flap misdiagnosed as recurrent tumor. Axial contrast-enhanced CT performed as the first posttreatment surveillance scan following resection of an SCC involving the lateral oral tongue, retromolar trigone, and lateral oropharynx. The enhancing nodular masslike lesion of the lateral oropharynx was mistakenly interpreted as recurrent tumor (arrow). After discussion with the otolaryngologist, this was determined to represent an SMG flap reconstruction of the lateral oropharynx.
Case Series
In this institutional review board–exempt and Health Insurance Portability and Accountability Act–compliant study, we retrospectively reviewed the records of a tertiary oncologic otolaryngology surgical practice performing pedicled locoregional glandulofascial flap reconstruction during 2014–2022, yielding 37 patients. We excluded all patients with no postoperative imaging and any patients in whom glands other than the SMG were used for the flap reconstruction (eg, thyroid glands mobilized to bolster the pharyngeal closure following laryngectomy). Nineteen patients met the inclusion criteria. All patients underwent an SMG flap operation with the intent of reconstruction and not transfer of the gland to shield it from high-dose radiation (ie, Seikaly and Jha submandibular transfer procedure).8
In total, 23 studies of SMG flaps were characterized, including 16 CTs and 7 MR images. Preoperative imaging was available for 14 patients, consisting of 12 CTs and 3 MR images. All CTs were performed with IV iodinated contrast and included multiplanar reconstructions. Of these, CT examinations performed at our institution included administration of 100 mL of iopamidol (Isovue; Bracco) using a split bolus technique of 60 mL contrast at 2.5 mL/s, a 35-second pause, 40 mL of contrast at 2.5 mL/s, 40 mL of saline at 2.5 mL/s, and scanning at 90 seconds after start of the injection. All MR imaging was performed without and with IV gadolinium-based contrast and consisted of, at a minimum, T1-weighted, T2-weighted fat-suppressed, DWI, and T1-weighted fat-suppressed postcontrast sequences. Of these, MR imaging examinations performed at our institution included administration of gadobenate dimeglumine (MultiHance; Bracco) per weight-based dosing.
RESULTS
In 19 patients, SMG flaps were used for reconstruction following primary resection of squamous cell carcinoma (SCC) of the oral cavity (n = 7), SCC of the oropharynx (n = 3), poorly differentiated carcinoma of the parotid (n = 2), parapharyngeal synovial cell sarcoma (n = 2), 1 case of metastatic SCC of unknown primary (p16 negative), and individual cases of mandibular ameloblastoma, deep lobe parotid pleomorphic adenoma, parotid adenoid cystic carcinoma, and parotid salivary ductal carcinoma. The time between the operation and imaging ranged from 1 month to 9 years, with a median follow-up of 7 months. SMG flaps were mobilized into the parapharyngeal space (n = 10), parotid bed (n = 4), marginal mandibulectomy defect (n = 3), and inferior to the mandibular body (n = 2). Once mobilized, the glands typically did not retain their usual glandular shape (n = 2), instead becoming distorted (n = 17) with triangular, fusiform, and overall ill-defined morphologies. There was variable CT enhancement, MR imaging enhancement, and MR imaging T2 signal intensity of the SMG flaps compared with the contralateral gland on postopertive studies or the preoperative ipsilateral gland. Most mobilized glands in the 23 studies had a heterogeneous appearance consistent with a subjective glandular texture (n = 14). The most common feature was a preserved glandular hilum, defined as visible ducts and/or a vascular pedicle contiguous with the gland (n = 19). In all cases, no distinct glandular tissue could be discerned at the orthotopic location of the SMG in the submandibular space.
DISCUSSION
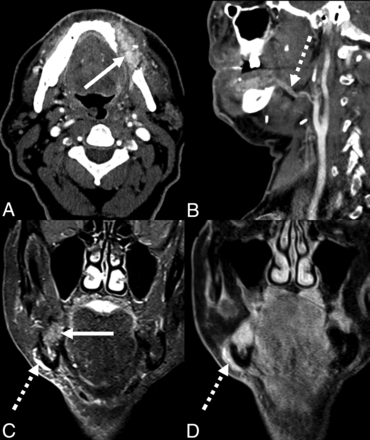
Imaging interpretation of the postoperative head and neck can be challenging for the radiologist, in part due to the diverse and complex surgical techniques encountered. To aid in interpretation, previous studies have described in detail the mobilization of glandular tissue, including the thyroid gland during laryngectomy and the SMG for glandular transfer. Therefore, this clinical report aimed to characterize the imaging appearance of the reconstructive SMG flap. In the surgical literature, the SMG flap has been described as an elegant reconstruction to facilitate closure of oropharyngeal defects or restore facial contour following parotidectomy (Fig 2) in instances in which primary closure may result in too much tension of the tissues, while a larger free flap would introduce further complexity of a microvascular operative technique.
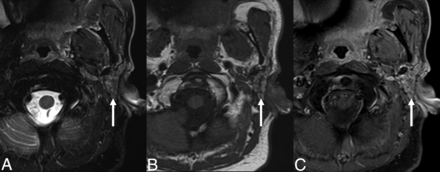
SMG flap of the parotid bed. Axial T2-weighted fat-suppressed (A), T1-weighted (B), and T1-weighted postcontrast fat-suppressed (C) MR images demonstrating facial contour reconstruction with an SMG flap in the parotid bed (arrow), after total parotidectomy for a poorly differentiated carcinoma.
The primary pitfall in imaging of SMG flaps in the postsurgical head and neck is that enhancing glandular tissue may be easily mistaken for recurrent tumor (Fig 1). In fact, this mistake was how such a surgical flap was brought to our attention, when a SMG mobilized to the lateral oropharynx was mistakenly interpreted as recurrent tumor. Certainly, in this setting, no adage is more appropriate than that no head and neck imaging interpretation is complete without a priori knowledge of the clinical and surgical history; knowledge of the existence of an SMG flap is of utmost importance for the radiologist. Preoperative imaging is also crucial to differentiate the intermediate enhancement typical of primary tumors from often hyperenhancing glandular tissue. However, recognizing the instances when our interpretations may be bereft of specific, relevant clinical information, we offer these characteristics that may aid the radiologist in recognizing the presence of such a flap (Fig 3).
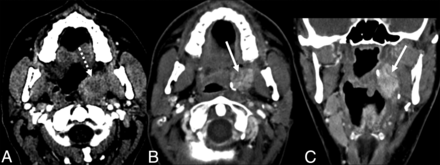
Typical SMG flap appearance. A, Axial contrast-enhanced CT of an SCC involving the left lateral oropharynx (dashed arrow). Axial (B) and coronal (C) contrast-enhanced CT images obtained 6 months after resection and reconstruction with an SMG flap show an enhancing, nodular, masslike lesion in the operative bed (solid arrow). The glandular heterogeneous enhancement, the semblance of a preserved hilum, and the absence of the native SMG in its orthotopic location in the submandibular space are useful in differentiating a normal SMG flap from recurrent tumor.
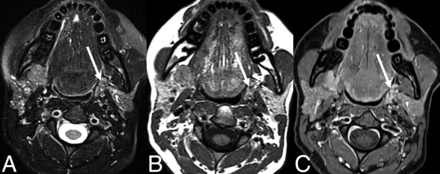
It may be logical to presume that a gland mobilized for a flap would maintain some semblance of its original imaging characteristics or similarity to the contralateral nonoperative gland. However, enhancement and T2 signal intensity in this series were unpredictable and, therefore, unreliable for gland identification (Fig 4). This issue is due to a variety of opposing factors. Edema and inflammation (eg, postsurgical, immediate postradiation, or localized sialadenitis) increase T2 signal intensity and enhancement. On the contrary, progressive atrophy (eg, long-term postradiation, postinflammatory, or sequelae of chronic ductal obstruction) decreases signal intensity on T2-weighted fat-suppressed and T1-weighted fat-suppressed postcontrast sequences. One notable exception was those glands that were atrophic and replaced by fat preoperatively and were invariably fatty in the postoperative period. A fat-replaced gland may introduce a countervailing interpretive pitfall, whereby nodular locally recurrent tumor may be falsely characterized as a normal SMG flap (Fig 5).
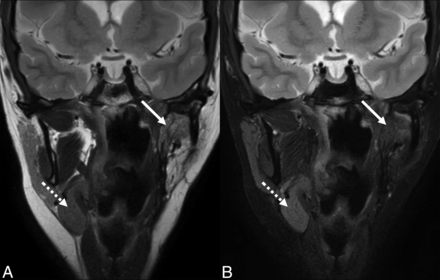
T2 signal intensity of an SMG flap. Coronal T2-weighted (A) and T2-weighted fat-suppressed (B) MR images of an SMG flap within the parapharyngeal space (solid arrow) for reconstruction following resection of a synovial cell sarcoma. MR imaging characteristics of SMG flaps are variable compared with the contralateral gland. Due to atrophy in this case, the mobilized gland is increased in signal intensity relative to the contralateral gland in the orthotopic location (dashed arrow) in the first image, but decreased in signal intensity in the fat-suppressed image.
A potential interpretation pitfall of SMG flaps. Axial (A) and coronal (B) contrast-enhanced CT images of a patient undergoing a first postoperative surveillance scan following marginal mandibulectomy and internal fixation for resection of an SCC of the retromolar trigone. The patient declined adjuvant chemoradiation. After reviewing the operative report, an enhancing masslike lesion medial to the mandible was presumed to represent the SMG flap (solid arrow). However, this enhancing tissue was later retrospectively revealed to be recurrent tumor adjacent to the SMG flap with an atrophic, fatty gland (dashed arrow). C, Axial contrast-enhanced CT from the preoperative staging study shows that the gland was originally low in attenuation due to fat content (dashed arrow). D, Axial contrast-enhanced CT of the second postoperative surveillance scan shows heterogeneous enhancement of progressive tumor (asterisk).
To the surgeon, the SMG flap is mobile and pliable, permitting placement in a wide range of useful locations. Thus, the radiologist can anticipate that the gland will largely conform to the surgical defect, depending on the volume of surrounding adipose tissue that is mobilized with the gland. For example, in this series, glands often conformed to the triangular shape of the parapharyngeal space, and those inferior to the mandible were elongated into a fusiform shape (Fig 6). The latter shape is similar to that described in the SMG transfer, yet it is the ipsilateral gland that is mobilized in a SMG flap, and the contralateral gland, for a SMG transfer.8 While it may seem intuitive, the absence of glandular tissue at the expected orthotopic location of the SMG may be the first clue to the radiologist that the gland has been manipulated, whether mobilized for reconstruction as in the case of the SMG flap or removed as part of the more frequently encountered neck dissection. Otherwise, features of a glandular hilum such as identifiable ducts and/or a vascular pedicle (Fig 7) and heterogeneous hyperenhancement of a glandular texture (Fig 8) are most useful in identifying a SMG flap and therefore differentiating it from recurrent tumor. As with any reconstructive flap, the margins should be closely evaluated as a site of potential recurrence, with care to differentiate tumor from the gland.
Distortion of the SMG flap into a fusiform shape. Axial contrast-enhanced CT following marginal mandibulectomy for resection of an ameloblastoma. The SMG flap appears as a fusiform enhancing nodule inferior to the operative bed (arrow). While similar in imaging appearance to a SMG transfer, this gland was mobilized for reconstruction of the ipsilateral surgical defect rather than to shield the contralateral gland away from the high-dose radiation treatment field.
SMG flaps and vascular pedicles in 2 patients following marginal mandibulectomy and SMG flap reconstruction for primary resection of an SCC of the oral cavity. Axial (A) and sagittal (B) contrast-enhanced CT images reveal the hyperenhancing, heterogeneous, glandular texture of the SMG flap (solid arrow) with a vascular pedicle including the facial artery coursing medial to the mandibular ramus (dashed arrow). Coronal STIR (C) and coronal T1-weighted fat-suppressed (D) postcontrast MR images show an SMG flap in the mandibulectomy defect (solid arrow) and vascular pedicle, including the facial vein coursing lateral to the mandibular body (dashed arrows).
SMG flap glandular texture. Axial T2-weighted fat-suppressed (A), T1-weighted (B), and T1-weighted postcontrast fat-suppressed (C) MR images in a patient with a pleomorphic adenoma of the parapharyngeal space following resection and reconstruction with an SMG flap (arrow). The gland conforms to the triangular shape of the parapharyngeal space, while maintaining a glandular heterogeneity similar to that in the adjacent parotid gland.
CONCLUSIONS
The SMG flap is a pedicled locoregional reconstruction flap occasionally used following oncologic resection within the head and neck. This feature presents a potential pitfall to the radiologist interpreting posttreatment head and neck examinations because an enhancing SMG flap can be confused with recurrent tumor. To reduce misdiagnosis, this clinical report raises awareness of this surgical technique and offers a description of the appearance of the SMG flap on CT and MR imaging.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 11, 2022.
- Accepted after revision February 21, 2023.
- © 2023 by American Journal of Neuroradiology