Abstract
SUMMARY: The Monro-Kellie doctrine is a well-accepted principle of intracranial hemodynamics. It has undergone few consequential revisions since it was established. Its principle is straightforward: The combined volume of neuronal tissue, blood, and CSF is constant. To maintain homeostatic intracranial pressure, any increase or decrease in one of these elements leads to a reciprocal and opposite change in the others. The Monro-Kellie doctrine assumes a rigid, unadaptable calvaria. Recent studies have disproven this assumption. The skull expands and grows in response to pathologic changes in intracranial pressure. In this review, we outline what is known about calvarial changes in the setting of pressure dysregulation and suggest a revision to the Monro-Kellie doctrine that includes an adaptable skull as a fourth component.
ABBREVIATIONS:
- IIH
- idiopathic intracranial hypertension
- SIH
- spontaneous intracranial hypotension
The Monro-Kellie doctrine has been a fundamental principle of neurophysiology for >200 years. Its hypothesis is based on simple reasoning: Within a rigid calvarial vault, the total volume of brain, CSF, and blood is constant. Any change in one of these elements results in an opposing compensatory response by the other 2 components.1 On neuroimaging, the effects of this doctrine are ubiquitous: Encephalomalacia leads to ex vacuo dilation of an adjacent ventricle, an edematous and enlarged brain compresses ventricles and vessels, and CSF hypovolemia results in pituitary and venous engorgement.
Multiple studies, however, now suggest that the Monro-Kellie doctrine is due for further examination, particularly in the setting of abnormal intracranial pressure. Both increased and decreased intracranial pressure exerts downstream effects on the calvaria. Increased pressure leads to calvarial thinning and pitting,2 while decreased pressure leads to development of layering hyperostosis along the inner table of the skull.3 These findings have substantial implications, both in terms of how we think of the Monro-Kellie doctrine and potentially how pathologies of intracranial pressure are identified and treated. In light of these findings, we review the history of the Monro-Kellie hypothesis and describe mounting evidence of a nonrigid, adaptable calvaria in the setting of pathologically elevated or reduced CSF pressure.
BACKGROUND
The Monro-Kellie doctrine underwent many early changes before achieving its current form. Alexander Monro, a Scottish doctor of impressive medical lineage, first proposed that a rigid skull contained an incompressible brain and a constant amount of blood; ie, a steady input of arterial blood led to a compensatory output in venous blood.4 In 1824, his student George Killie de Leith provided postmortem evidence of this theory. Neither, however, mentioned CSF.5 Around the same time, John Ambercrombie made similar observations in animals; some early citations of this theorem were entitled the “Monro-Abercrombie doctrine.”6
George Burrows first included CSF in this model, and it was not until 1926 that Harvey Cushing7 neatly summarized the Monro-Kellie doctrine as it is generally known today. According to Cushing, the “three elements” (blood, nervous tissue, and fluid) in the skull “must always remain the same in bulk….any increase in blood volume, for example, can only take place at the expense of one of the other elements.” Cushing considered the responsiveness of any reciprocal action to be prompt, “These changes all take place rapidly—a matter of minutes.”7
Of course, self-evident exceptions to this rule abound. A foreign body may enter the calvarial vault, displacing or decreasing all 3 native intracranial components. Many types of intracranial masses are not composed of brain, CSF, or blood (eg, abscesses, metastases, and granulation tissue). These exceptions do not require a revision of the doctrine because they neither challenge the underlying mechanism on which the Monro-Kellie doctrine is built nor change the primary assumption of a fixed-volume skull.
Our understanding of intracranial pressure dynamics has become more nuanced since the inception of the doctrine. It is now known, for example, that blood plays a much greater role in dictating intracranial pressure than CSF. The total intracranial in- and outflow of blood is approximately 700 mL/min, occupying 100–130 mL of intracranial volume at any time. CSF, conversely, is produced at only 0.35 mL/min, with a total volume of about 75 mL.5 The cardiac cycle also dynamically influences intracranial pressure, with variations in arterial input during systolic and diastolic flow resulting in pulsatile brain movement, brain expansion and contraction, and intraventricular CSF flow.8
Although our understanding of intracranial pressure has become more refined, the Monro-Kellie doctrine has notably not been adjusted or revised. A few prior studies have proposed minor updates regarding concepts such as tissue elasticity and compliance9 and the dynamic nature of intracranial arterial and venous vascularity.5 None of these proposals have strayed far from the tenets of the doctrine: 1) The 3 major constituents that play a role in intracranial pressure homeostasis are blood, CSF, and brain parenchyma, and 2) the calvaria is rigid and has a fixed volume.
The closest proposed revision came from Mascarenhas et al,6 who performed a set of postmortem experiments in which they expanded a rubber balloon in a skull while measuring skull deformation. The results indicated that deformation was noted during increases and decreases in internal pressure. The authors consequently contended that the Monro-Kellie doctrine was, therefore, invalid because it failed to acknowledge such deformational changes in the skull.
Now there is convincing evidence of a more chronic type of elasticity of the calvaria in response to increases and decreases in intracranial pressure. Unlike the experiments of Mascarenhas et al,6 however, this elasticity manifests as changes in the thickness and shape of the calvarium, rather than transient deformations. Both types of changes represent adaptivity of the skull to expand or contract the calvarial vault in response to CSF pressure abnormalities. Below, we review in detail what is currently known about such calvarial changes.
Intracranial Hypertension
Intracranial hypertension was first described in 1893 by Heinrich Quincke, a disease he labeled “meningitis serosa” attributed to alterations in CSF secretions.10 In 1904, Max Nonne first used the term pseudotumor cerebri (“false brain tumor”) to describe this syndrome. Nonne’s intent was to propose a state of elevated intracranial pressure that was distinct from that related to cerebral tumors: intercranial hypertension that followed a less sinister course.11 John Foley12 later offered the term “benign intracranial hypertension.”
These labels have become outdated. First, several other etiologies of increased intracranial pressure have become known. Such cases are referred to as secondary intracranial hypertension, reserving the term “primary pseudotumor cerebri” (also known an idiopathic intracranial hypertension [IIH]) for cases of cryptogenic elevated intracranial pressure. Next, the vision loss associated with IIH has made the label “benign” inappropriate.13
The etiologic mechanism for IIH remains unknown. Theories generally fall into the categories of altered CSF hydrodynamics or hindrances to venous outflow.14 The disorder represents a dysfunction in the homeostatic regulatory role of the Monro-Kellie doctrine; the increase in ≥1 intracranial component is insufficiently balanced by a corresponding decrease in the others.15
On imaging, numerous sequelae of IIH have been well-established. The pituitary gland is typically flattened along the floor of the sella, thought to be related to downward herniation of an arachnocele through the diaphragma sella. Ventricles are often slitlike.16 Many findings have been reported in the orbits: flattening of the posterior sclera, distention of the perioptic subarachnoid space, tortuosity of the optic nerves, and intraocular protrusion of the optic nerve.17 Most important, lateral transverse sinus stenosis is also common, likely mediated by and contributing to increased intracranial pressure.18
Recent studies have demonstrated that remodeling of the calvaria is common in the setting of chronic pseudotumor cerebri. In such patients, the elevated intracranial pressure can lead to enlargement of the sella turcica (Fig 1),19 as well as prominent arachnoid pits and/or arachnoid granulations.2 Meningoceles are also common, typically forming in the temporal bone (Fig 2).20 As Bialer et al20 noted, these findings are all biomechanically similar in that they increase the volume of the subarachnoid space, thereby at least conceptually decreasing intracranial pressure. Skull base erosion and meningocele formation can lead to the development of CSF leaks.21 In some cases, encephaloceles in these locations may also lead to epilepsy.22
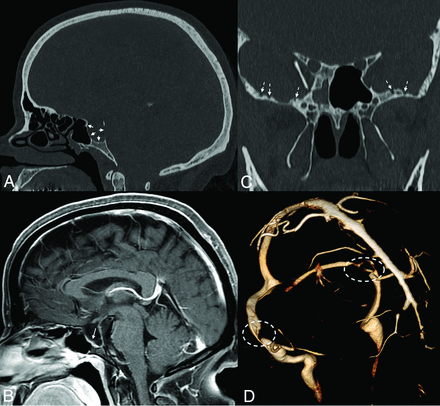
Sella expansion and skull base pitting in IIH. Sagittal CT image (A) in a 38-year-old woman with pseudotumor cerebri demonstrates marked expansion of the osseous walls of the sella (short solid arrows), with frank dehiscence posteriorly. Corresponding sagittal MR image (B) shows flattening of the pituitary tissue along the floor of the sella (long solid arrow). Prominent pitting is also noted along the anterior margins of both middle cranial fossae (dashed arrows, C). 3D reconstruction image of an MRV (D) demonstrates smooth tapering of the bilateral transverse sinuses (dashed ovals), compatible with IIH.
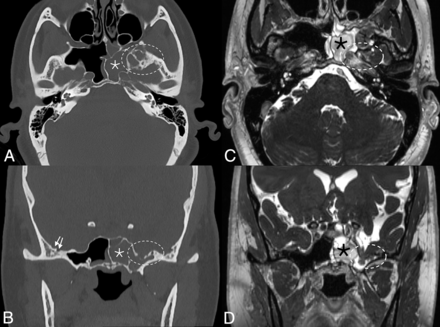
Skull pitting, meningoceles, and CSF leak in a patient who presented with rhinorrhea. Axial and coronal CT images (A and B) demonstrate substantial pitting along the anteromedial aspect of the left middle cranial fossa (dashed ovals, A and B), with complete opacification of the left sphenoid sinus (asterisks), concerning for a CSF leak. Milder pitting is noted on the right (solid arrows). Corresponding MR imaging (C and D) confirms a meningocele protruding into the left sphenoid sinus (dashed ovals, C and D). MRV (not shown) noted smooth tapered stenoses involving both transverse sinuses, and the patient was ultimately diagnosed with pseudotumor cerebri.
Beyond distinct areas of pitting, the skull itself becomes thinned in IIH. A case-control study by Barke et al23 found both skull base thickness (P < .001) and calvarial width (P = .024) to be significantly smaller in patients with IIH than in controls. Rabbani et al24 confirmed such findings, noting that while advancing age was typically associated with increased calvarial thickness, increased age portended calvarial thinning in patients with IIH. These calvarial changes are in opposition to the conventional understanding of the Monro-Kellie doctrine.
Except for CSF leaks and rare cases of epilepsy related to encephaloceles, little remains known about the clinical relevance of calvarial expansion and/or thinning in the setting of IIH. To date, no studies have shown that these calvarial changes affect patient outcomes. Still, IIH remains an evolving field. As our knowledge of this disorder continues to grow, it is possible that calvarial changes among some patients with IIH could affect treatment strategies or could be used as a prognostic marker.
Intracranial Hypotension
Spontaneous intracranial hypotension (SIH) is related to CSF volume depletion. Although CSF leaks may be secondary to trauma and iatrogenic causes such as surgery or lumbar punctures, intracranial hypotension by fiat is due to spontaneous spinal CSF leaks.25 Like IIH, the intracranial sequelae of intracranial hypotension have been well-described on imaging.
Even more so than intracranial hypertension, most of the intracranial sequelae of SIH can be explained by the conventional understanding of the Monro-Kellie doctrine. For example, the relative dearth of CSF results in pituitary enlargement (ie, opposite to IIH) and expansion of the dural venous sinuses.26,27 The dura engorges, leading to diffuse dural thickening and enhancement.28 In some patients, subdural fluid collections, either hygromas or hematomas, develop over the cerebral convexities.29 On the basis of the presence or absence of these findings, one can reliably predict the likelihood of a patient having SIH.29 Ultimately, SIH represents a disorder in which autoregulatory mechanisms are insufficient to compensate for the CSF loss, and other imaging abnormalities reflect this breakdown. Specifically, the brain begins to sag inferiorly, with effacement of the suprasellar and prepontine cisterns and decreased mamillopontine distance.29
Despite the number of changes observed, most of the compensatory changes in SIH are related to hyperemia; other than the pituitary gland, the brain parenchyma is relatively unable to expand in response to CSF depletion.30 The same is true following treatment. Once a leak has been repaired, intracranial CSF volume has been shown to increase significantly over its hypotensive baseline. The brain volume in the posttreatment setting, in contrast, remains the same.17,31 Thus, the main compensatory action following CSF leak repair is a reciprocal reduction of intracranial blood volume.
It is only recently that we have become aware of calvarial changes in the setting of SIH. Johnson et al32 first showed that patients with SIH were often observed to have a thickened calvaria, often with a characteristic layered hyperostotic growth pattern along the inner table of the skull. In the setting of long-standing SIH, the authors opined, calvarial growth served as an additional compensatory mechanism for the depleted intracranial CSF volume. Babcock et al3 confirmed such findings in a case-control study, noting that layered hyperostosis was present in 32% of patients with SIH but in only 5% of controls (P < .001; OR = 11.58) (Fig 3). Most important, the study of Babcock et al found no significant difference in the prevalence of diffuse (nonlayered) hyperostosis between groups (P = .465). Thus, the “layer cake” type of hyperostosis is much more indicative of intracranial hypotension (Fig 4). Although the expected location of this layer cake appearance was not discussed in detail in either publication, it is possible that these findings tend to be frontal-predominant, similar to benign hyperostosis frontalis interna. Specifically, the representative examples in the article by Babcock et al seem to suggest that such findings are at least prone to more substantial development along the frontal bone.
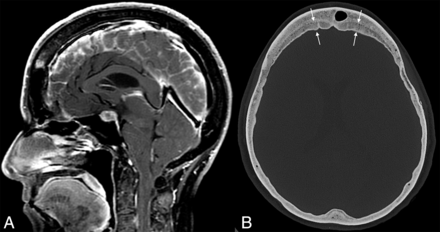
Example of layered calvarial thickening in a patient with SIH. Sagittal MR images (A) demonstrate classic findings of SIH, including pituitary enlargement, brain sag, and dural venous sinus engorgement. Axial CT image (B) in the same patient shows thickening of the bifrontal calvaria with characteristic layering related to preferential growth along the inner table of the skull (between straight arrows).
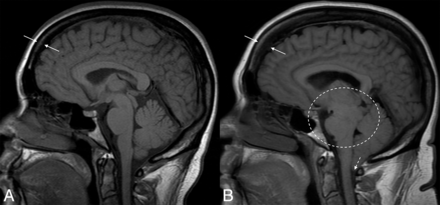
Hyperostosis related to a CSF leak. Sagittal T1-weight images before (A) and after (B) the development and diagnosis of SIH, with 16 years between examinations. Multiple classic findings of SIH are seen, including brain sag (dashed oval) and herniation of the cerebellar tonsils through the foramen magnum (dashed arrow). The patient also developed substantial frontal-predominant calvarial hyperostosis (between solid arrows), particularly along the inner table of the skull.
A similar phenomenon, termed “hyperostosis cranii ex vacuo” has been described in chronic shunting of pediatric hydrocephalus.33,34 In this condition, there is prominent growth of histologically lamellar bone, with scattered foci of woven bone.33 This growth, Moseley et al35 noted, is primarily the inner table of the calvaria. Thus, the findings are analogous to the layer cake appearance seen in adult patients with SIH. The increased inner-to-outer table measurement is important because it helps distinguish these calvarial changes from those related to expansion of the diploic space (eg, sickle cell disease or thalassemia).33
The clinical implications of these calvarial findings are currently unknown. There are, however, some potential effects that have been considered. The presence of layer cake hyperostosis in patients with SIH, for example, leads to a decrease in the volume of the calvarial vault. Conceptually, this could reduce the ability to normalize brain sag following CSF leak repair—that is, calvarial hyperostosis could impede the ability of brain to “un-sag” by acting as an unyielding intracranial barrier. Similarly, patients with SIH with layer cake skull changes might be at higher risk of rebound hypertension after leak repair because the tightened intracranial space could potentially lead to greater mass effect on the transverse sinuses. For now, these hypotheses remain conceptual in nature, though studies on these topics are ongoing.
Call For Revision
Altogether, studies have convincingly shown demonstrable changes in the calvaria in the setting of pathologic conditions of intracranial pressure. The skull expands in response to IIH and grows inward along its inner table in response to SIH. Skeptics might argue that these changes are outside the realm of the Monro-Kellie doctrine because they represent adaptive changes in response to a breakdown of pressure homeostasis. It is clear, however, that the concept of a rigid calvaria, a fundamental tenet upon which the doctrine is based, has been disproven multiple times. We should add a fourth component to the Monro-Kellie hypothesis: a nonrigid calvaria that adjusts, albeit slowly, to changes in intracranial pressure.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received August 27, 2022.
- Accepted after revision October 5, 2022.
- © 2023 by American Journal of Neuroradiology