Abstract
BACKGROUND AND PURPOSE: The quality of leptomeningeal collaterals may influence the speed of infarct progression in acute stroke. Our main objective was to evaluate the association of leptomeningeal collateral score and its interaction with time with ischemic changes on CT in patients with acute stroke.
MATERIALS AND METHODS: Adult patients with acute stroke symptoms and anterior circulation large-vessel occlusion on CTA from 2015 to 2019 were included. Routinely performed NCCT and multiphase CTA were reviewed to assess ASPECTS and the leptomeningeal collateral score. We built multivariate regression models to assess the association between leptomeningeal collateral score and its interaction with time and ASPECTS. Performance measures to predict poor ASPECTS at different time thresholds (identified with receiver operating characteristic curve analysis) were estimated in a subgroup of patients with poor leptomeningeal collateral scores.
RESULTS: Leptomeningeal collateral scores 0–1 were associated with lower ASPECTS, and the model with dichotomized and trichotomized leptomeningeal collateral score showed a significant multiplicative interaction between time and the leptomeningeal collateral score. The negative predictive value for poor ASPECTS was >0.9 for at least the first 3 hours from stroke onset to imaging, and the positive predictive value was <0.5 for every time threshold tested in the subgroup of patients with leptomeningeal collateral scores 0–3.
CONCLUSIONS: Poor (0–1) leptomeningeal collateral scores were associated with lower ASPECTS, and an increase in time has a multiplicative interaction with the leptomeningeal collateral score on ASPECTS.
ABBREVIATIONS:
- CollS
- leptomeningeal collateral score
- mCTA
- multiphase CTA
- NPV
- negative predictive value
- PPV
- positive predictive value
- ROC
- receiver operating characteristic
Endovascular therapy decreases the risk of disability in patients with acute ischemic stroke due to anterior circulation large-vessel occlusion, particularly if the ischemic core is small enough compared with the rest of the hypoperfused brain.1-6 Because the ischemic core tends to increase with time before revascularization is achieved, a delay between stroke onset and revascularization influences the efficiency of the treatment. However, some patients may still benefit from endovascular treatment as long as 24 hours after stroke onset.3-6 This benefit includes patients living in remote areas who may be considered if the progression of the ischemic core is slow enough.7 The ability to predict ischemic core progression in these patients has the potential to assist in their selection for transfer to stroke centers. However, predicting which patients will progress at which rate is still a challenge.8
Leptomeningeal collateral status may be a key element in this important decision-making process. The role of collaterals in acute stroke has been an important research topic and a subject of numerous publications during the past few years. Good collaterals are associated with a better functional outcome for patients with acute ischemic stroke,9-12 especially in the context of endovascular therapy,13-17 and more recently when considering endovascular therapy in patients with a low ASPECTS.18,19
Other authors have been focusing on radiologic parameters and the association among collaterals, the infarct core, and the infarct growth rate. Previous findings have shown that the ASPECTS and leptomeningeal collaterals may provide an indirect evaluation of the infarct core volume for a selection of patients with acute ischemic stroke and therefore assist in patient selection for endovascular therapy in an extended time window.20-22 More recently, secondary analysis from the Randomized controlled trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study showed that the early infarct growth rate strongly correlates with both collateral status and clinical outcomes after endovascular thrombectomy.23 Regenhardt et al,24 in 2022, also recently correlated symmetric collaterals with a low ischemic core growth rate. However, the relationship between leptomeningeal collaterals and the infarct core growth rate remains poorly understood with contradictory results in the literature25-28 and lack of a thorough analysis of the effect of the interaction between time and leptomeningeal collaterals on ischemic core volume.
To evaluate the relation of leptomeningeal collaterals and time with infarct core volume in a pragmatic manner that reflects the real-life trajectories and resources of community centers, we conducted a single-center retrospective study, using the ASPECTS as a surrogate for the ischemic core volume. In this study, we aimed to analyze the effect of the leptomeningeal collateral score (CollS) and its interaction with time from stroke to imaging on the ASPECTS. We also sought to find a time threshold having the best performance values to predict ASPECTS, in patients with poor CollS.
MATERIALS AND METHODS
Design
We report this study in accordance with the REporting of studies Conducted by using Observational Routinely collected health Data (RECORD) statement.29 The study received approval from our local institutional review board, and the need for patient consent was waived because data were exclusively collected on medical charts.
Setting
This study was conducted in a comprehensive stroke center located in Quebec city, Canada. All included patients underwent multiphase CTA (mCTA) for collaterals. Patients were identified through our radiology database (PACS) using a specific code related to acute stroke, from May 25, 2015 (on implementation of the mCTA protocol), to May 24, 2019. The entire protocol for patients with stroke includes an NCCT as well as mCTA. No data on perfusion studies were collected because CT perfusion was not available at our center at the above-mentioned dates.
Participants
We screened all consecutive patients admitted for acute ischemic stroke. The selected patients were all adults 18 years of age or older with acute stroke symptoms and large-vessel occlusion of the anterior cerebral circulation, including the ICA and proximal middle cerebral artery (M1 or proximal M2). Patients with either no occlusion, distal anterior circulation occlusion (distal M2 and A2), posterior circulation occlusion, or hemorrhagic stroke were excluded from the study.
Variables and Data Sources
The ASPECTS was evaluated independently by 1 vascular neurologist and 1 neurosurgeon with endovascular training. The assessment was made according to the guidelines published by the team who created this score, and both reviewers received appropriate training.7,30 A third party (a vascular neurologist) was consulted in case of disagreement. This assessment was blinded to the quality of CollS and to all clinical data, including the time from stroke to imaging.
The assessment of collateral status on mCTA was performed separately and independently by 2 interventional neuroradiologists with a score ranging from 0 to 5 according to Menon et al.31 A third party (a neurosurgeon specialized in neurointervention) reviewed all disagreements. If the third reviewer was not in agreement with one of the first 2 reviewers, the disagreement had to be resolved through discussion. This measurement was also blinded to clinical data. Because the CollS evaluation had to be independent of the ASPECTS, the investigators were required to mention all patients for whom a bad ASPECTS from large stroke could have been deduced on CTA images. The CollSs were trichotomized as poor (0–1), intermediate (2–3), and good (4–5) and dichotomized as poor (0–3) and good (4–5) for analyses.
Clinical data, including age, sex, NIHSS, time from stroke to imaging, smoking status, intravenous thrombolysis and thrombectomy status, and anticoagulant, antiplatelet, antihypertensive, lipid-lowering, and hypoglycemic drug use, were collected separately and independent from electronic medical records by 2 members of the team. For patients with no precise time from stroke onset, the time when the patient was last-seen-well was used. When the NIHSS was not available from the medical record, a retrospective scoring algorithm was used to estimate it.32 Discrepancies were resolved through discussion.
The time from stroke to imaging was calculated afterward as the duration between the time of stroke onset (or last seen well) and the time of CT/CTA of the brain. All data collectors were blinded to the exact time from stroke to imaging.
Statistical Analyses
Sample size calculation was based on parameters issued from preliminary data collected from the first 23 patients. The mean (SD) of the time from stroke to imaging (2.3 hours and 1.74 hours, respectively) represented 60% of the bad ASPECTS in the poor CollS group. A sample size of ≥145 cases was sufficient to obtain 80% power in a multivariate logistic regression model with a conservative OR of 1.4 for the CollS factor. Furthermore, 10% of R-square was attributed to the time from stroke to imaging, and the significance level was set at .05. Considering the possible 20% of missing data, the final sample size was set at 175 cases.
Quantitative variables are described as mean (SD) and median, first (Q1) and third (Q3) quartiles, minimum, and maximum; qualitative variables are described as frequencies and percentages.
Interobserver agreement for ASPECTS and CollS data was measured with the Cohen κ for dichotomized and trichotomized data. The intraclass coefficient correlation was used to assess agreement with the original scales. The κ coefficient was defined as slight (0–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or excellent (0.81–1).33 The intraclass correlation coefficient was categorized as follows: poor (<0.50), moderate (0.50–0.75), good (0.75–0.90), and excellent (>0.90).34
Receiver operating characteristic (ROC) curve analyses were used to find time thresholds from stroke to imaging having the best performance values to predict bad ASPECTS (0–5) in 2 subgroups of CollS (0–1 and 0–3). The performance measures of the different time thresholds for bad ASPECTS were estimated with asymptotic 95% CIs. For ROC curve analysis, the ASPECTS was dichotomized with scores ranging from 6 to 10 qualifying as good and scores and from 0 to 5 qualifying as bad. The threshold of ≥6 was chosen because it is an inclusion criterion for thrombectomy in the guidelines.35,36
The association of CollS and its interaction with time on ASPECTS was analyzed in multivariate linear regression models, entering CollS either as a continuous variable (model 1) or after dichotomization (model 2) or trichotomization (model 3). The time from stroke to imaging, age, NIHSS, intracranial occlusion location, the presence of tandem carotid occlusion, and an interaction term between time and the CollS were also entered in all models.
Statistical analyses were performed using SAS Statistical Software, Version.9.4 (SAS Institute) with a 2-sided significance level set at P < .05.
RESULTS
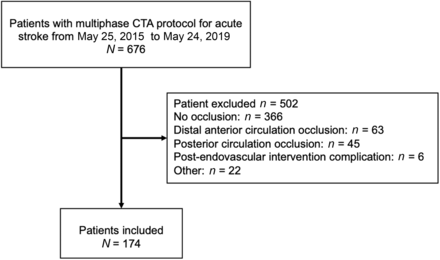
According to the radiologic database, 676 patients underwent mCTA at our institution between May 25, 2015, and May 24, 2019, and 174 patients were included in the analyses (Fig 1). Patients’ demographic and clinical data are shown in the Table. Most the patients’ CollSs were about equally distributed between 4 and 5 (n = 81; 46%) and 2 and 3 (n = 82; 47.1%). Very few patients had CollSs of 0–1 (n = 11; 6.3%). Poor CollSs were found in 10% (4/40) of ICA occlusions, 4.21% (4/95) of M1 occlusions, and 7.69% (3/39) of M2 occlusions.
Flow chart of patient selection.
Demographic and clinical characteristics of included patients (n = 174)
The estimated simple κ for the CollS was 0.41 (95% CI, 0.27–0.54) after dichotomization and 0.42 (95% CI, 0.30–0.54) after trichotomization, while the intraclass correlation coefficient was 0.57 (95% CI, 0.46–0.66). The estimated simple κ for ASPECTS was 0.60 (95% CI, 0.42–0.78), and the intraclass correlation coefficient was 0.69 (95% CI, 0.61–0.76). The agreement when using a cutoff at 5 instead of 6 to dichotomize ASPECTS was only 0.26 (95% CI, 0.01–0.53).
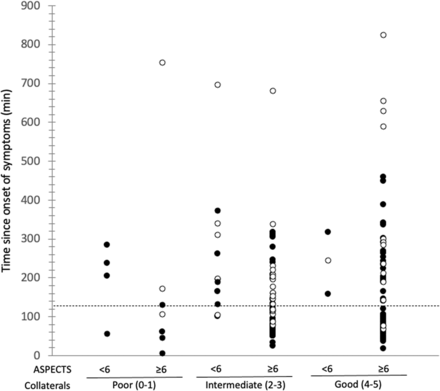
Figure 2 shows the distribution of our cohort according to the CollS, ASPECTS, and time from stroke to imaging. Among patients with witnessed stroke onset and good collaterals (CollS = 4–5), good ASPECTS (6–10) were seen as late as 448 minutes (7.5 hours) after stroke onset, but bad ASPECTS (0–5) were also seen as soon as 158 minutes after stroke onset.
Time distribution since stroke onset in the stratified study population. Patients with witnessed time from stroke onset are represented as solid circles, and patients classified as last seen well or with wake-up stroke are represented by open circles.
As shown in the Online Supplemental Data, in multivariate analysis, CollS 0–1 was independently associated with lower ASPECTS compared with CollS 4–5. The interaction term between poor CollS and time was also independently associated with ASPECTS in patients with CollS 2–3 and 0–3. For every 10-minute increase in time from stroke to imaging, there was a decrease in the ASPECTS of 0.07 (95% CI, 0.1–0.05; P < .001) in patients with CollS 2–3 and 0.06 (95% CI, 0.03–0.08; P = .001) in patients with CollS 0–3. In other words, a 1-point decay in the ASPECTS occurred for every 135 minutes (95% CI, 100–200) in the subgroup of patients with CollS 2–3 and for every 167 minutes (95% CI, 125–333) in the subgroup of patients with CollS 0–3.
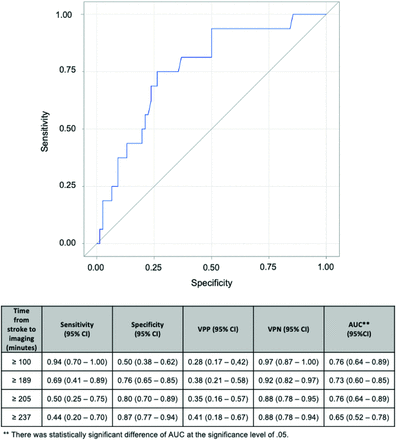
According to ROC curve analyses (Fig 3), the time threshold for predicting poor ASPECTS (0–5) with the highest negative predictive value (NPV) was 100 minutes (NPV = 0.97; 95% CI, 0.87–0.99) in the instance of CollS 0–3. The NPV remains above 0.90 (95% CI, 0.82–0.97) for the time threshold of <190 minutes and 0.88 (95% CI, 0.78–0.94) for time thresholds of <236 minutes. The positive predictive values (PPVs) were <0.5 for all time thresholds in this subgroup. The subgroup of CollS 0–1 was too small to perform accurate ROC curve analyses and allow precise estimation of the predictive values.
ROC curves for determining best performances measures to predict bad ASPECTS at different time values. AUC indicates area under the curve.
DISCUSSION
In our study, a poor CollS (0–1) was independently associated with lower ASPECTS as was the multiplicative interaction of time with CollS. The NPV to predict poor ASPECTS was >0.9 for at least the first 3 hours from stroke onset to imaging, and the PPV was <0.5 for every time threshold in the subgroup of patients with CollS 0–3.
The association of ASPECTS and collaterals has been reported in many studies during the past decade, with varying results. While post hoc analysis of the Interventional Management of Stroke (IMS III) and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE) 3 trials did not show a significant difference among patients according to their collateral status,10,26 other studies have shown a protective effect of good collateralization.37,38 Results from a retrospective cohort study suggested that collaterals can influence early ischemic changes; patients transferred from regional hospitals with poor collaterals were significantly more at risk of ASPECTS decay than those with good/intermediate collaterals (OR = 5.14; 95% CI, 2.20–12.70).39 Recent studies have shown that collateral status had the strongest association with infarct growth rate, while time from stroke onset did not reach statistical significance.27,28,40 In our study, we found that poor collaterals (0–1) were significantly associated with lower ASPECTS. On the other hand, some patients in our cohort who had imaging as soon as 100 minutes from stroke onset had bad ASPECTS despite CollS 4–5, suggesting that good collaterals alone do not guarantee a slow infarct growth rate. A similar observation was made in a post hoc analysis of the Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times (ESCAPE) trial, in which some fast-progressing patients had good collaterals.41
Finding a time threshold to select patients for treatment or to predict outcomes in acute ischemic stroke is still a challenge. The 6-hour threshold is used as the limit to discriminate between fast and slow progressors,42 but most of our patients presented within this threshold. Therefore, we sought to identify a time threshold of patients with a poor CollS that would distinguish fast and very fast progressors. Given that the consequences of excluding a patient with stroke who was indeed eligible for endovascular therapy (good ASPECTS after a certain time threshold) are far worse than including a patient with stroke who is not eligible (bad ASPECTS within a certain time threshold), the ideal time threshold would offer the highest possible PPV. All time thresholds analyzed (within 4 hours) offer a low PPV in patients with CollS 0–3, meaning that, on the basis of our results, it was not possible to identify a time threshold after which patients with CollS 0–3 were more likely to have poor ASPECTS.
On the other hand, the NPV remains high for a long time despite CollS 0–3, meaning that the risk of including poor ASPECTS despite CollS 0–3 remains low within at least 3 hours. Indeed, the effect of time on the ASPECTS found in our study showed that it requires 135 minutes to decrease by only 1 the ASPECTS in patients with CollS 2–3, possibly suggesting that lower CollSs are responsible for ischemic core progression leading to poor ASPECTS on a time threshold after ≥6 hours and that it is not possible to distinguish very fast progressors on the basis of CollS 0–3 alone. Finally, poor CollS 0–1 may cause faster ischemic core progression, but a larger sample size with larger distribution of time from stroke to imaging is required to analyze the interaction with time in this subgroup.
In our study, 2 patients had ASPECTS of 5 after 130 minutes despite poor CollS 0–1. Using 5 instead of 6 as a cutoff to include patients for mechanical thrombectomy is an ongoing debate.27,35,36,40-42 However, according to our results (κ = 0.2 for dichotomized ASPECTS at 5), it appears difficult to discriminate between ASPECTS 0–4 and ASPECTS 5. Using a cutoff of 5 in our cohort could have wrongly included a bad (0–4) ASPECTS as a good ASPECTS. In a review article, authors mentioned the variable interobserver agreement, going from fair to good, for dichotomized ASPECTS at 7, but the agreement for lower ASPECTS dichotomization is less well-established in the literature.43
The interobserver agreement for CollS is only fair-to-moderate and is much lower than the one reported by the team that created the Calgary Collateral Score (κ coefficient = 0.81).31 This difference may suggest a lack of external generalizability because we can expect that the team that created a radiologic score had a higher agreement than ours. Additionally, there are many examples of low agreement among readers assessing collaterals, even in the case of expert neuroradiologists.30,44-46 These examples show that every physician involved in acute ischemic stroke care should, therefore, be very careful when excluding patients on behalf of a single radiologic interpretation. This suggestion places emphasis on the importance of specific training such as what already exists with the ASPECTS (http://aspectsinstroke.com/), even for experienced neuroadiologists and may be another argument in favor of computer-aided triage in acute stroke.45
The main limitation of our study is its retrospective and cross-sectional design. The ideal design to assess the association between ASPECTS and CollS and its interaction with time would have been a prospective longitudinal design and would have required multiple radiologic examinations on a single patient at different time points and the transfer of all outside patients to our center—even if not eligible for thrombectomy—to perform an mCTA. Such a design would have been ethically questionable. Furthermore, because the images were obtained at only 1 time point, we implicitly assumed that the CollS does not change with time. Proving this point would have required multiple examinations during a short time, which are not routinely performed in our center and could add unnecessary delays to proper patient treatment.
Our design could also have been vulnerable to selection bias because we included transferred patients who were previously judged as potential candidates for thrombectomy, while those who were not eligible stayed at their referring center and were, therefore, excluded from our sample. Although this design should not have biased our results, it precluded us from studying the effect of collateral status with a longer delay. Also, our study has a low number of patients with CollS 0–1 (n = 11, 6%). This number is at the lowest limit of what is described in the literature, in which, depending on the scale, between 5% and 36% of the patients were identified with poor collaterals.47,48 Less than 11% of our patients presented with bad ASPECTS (≤5). Although this is a relatively small proportion of our cohort, it is still within the range found in the literature in which some cohorts had as low as 5% of bad ASPECTS.49 The radiologic reviewers were completely blinded to the time from stroke onset and other clinical and radiologic data. However, the ASPECTS was deduced by mCTA readers in 5 patients overall, but no significant change was found when carrying out sensitivity analysis excluding these data. The inclusion of unwitnessed stroke onset in our study may have biased the performance measures of a higher time threshold toward higher NPV and biased the time required to lose 1 point on ASPECTS toward higher estimates. Finally, our study shows good external validity because it was pragmatic and reflects real life with simple radiologic data that stroke clinicians must consider every time they decide on acute ischemic stroke management. It also reinforces the hypothesis of a relationship between faster ischemic changes and poor collateral status, which is frequently assumed and deliberately sought in only a few studies in literature.25,28,50
CONCLUSIONS
Poor (0–1) CollSs were associated with lower ASPECTS, and an increase in time has a multiplicative interaction with CollS on ASPECTS. Patients presenting within 4 hours may still have a good ASPECTS despite CollS 0–3, given the high NPV of this time threshold in this subgroup. Transferred patients with poor and intermediate collaterals should be rescanned at arrival in case of more rapid ASPECTS decay with time, to support endovascular therapy decisions. Finally, good collaterals alone are not a guarantee of a slow infarct growth rate, and moderate interobserver agreement for CollS mandates special attention—particularly when excluding patients on the basis of this criterion alone.
Footnotes
This study was funded by an educational grant from Stryker.
Disclosures: Marie-Éve Audet—RELATED: Grant: Stryker, Comments: Educational grant.* Jean-Luc Gariépy—RELATED: Grant: Stryker, Comments: Educational grant.* Pascale Lavoie—RELATED: Grant: Stryker, Comments: Educational grant.* Marie-Christine Camden—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Bristol-Myers-Squibb.* Steve Verreault—UNRELATED: Grants/Grants Pending: Bristol Myers Squibb, Portola; Public Health Research Institute; Payment for Lectures Including Service on Speakers Bureaus: Servier, Bristol Myers Squibb, Pfizer.* Money paid to the institution.
References
- Received February 10, 2021.
- Accepted after revision July 13, 2022.
- © 2022 by American Journal of Neuroradiology