Abstract
Phosphaturic mesenchymal tumors (PMTs) are neoplasms associated with tumor-induced osteomalacia. Patients typically present with pathologic fractures in the setting of chronic hypophosphatemic hyperphosphaturic osteomalacia, as well as gradual muscle weakness, bone pain, and difficulty walking. Because of their rarity and nonspecific symptomatology, phosphaturic mesenchymal tumors often go undiagnosed for years. Even when discovered on imaging, the tumors can be diagnostically challenging for radiologists. Phosphaturic mesenchymal tumors often tend to be small and can be located nearly anywhere in the body, and, therefore, can mimic many other tumors. This case highlights the imaging and pathologic markers of a phosphaturic mesenchymal tumor, often found in a patient with tumor-induced osteomalacia.
ABBREVIATIONS:
- FGF23
- fibroblast growth factor 23
- PMT
- phosphaturic mesenchymal tumor
- TIO
- tumor-induced osteomalacia
The patient is a 57-year-old man who presented to our institution for evaluation with a 4-year history of recurrent insufficiency fractures involving his femurs and ribs, associated with considerable disability. His initial symptom was right-hip pain that failed to resolve with conservative treatment. An MR imaging at that time showed a left subtrochanteric insufficiency fracture. Two years later, after developing pain in both thighs, the patient was diagnosed with insufficiency fractures involving the subtrochanteric regions of both proximal femurs. At the time of presentation, a review of his laboratory records revealed chronic hypophosphatemia, with serum phosphorus levels ranging between 1.7 and 2.1 mg/dL during the preceding 4 years. His serum calcium and parathyroid hormone levels have been consistently within the normal range.
Given his history and laboratory abnormalities, the diagnosis of tumor-induced osteomalacia (TIO) was entertained. Confirmatory testing (Table) demonstrated hypophosphatemia due to renal phosphate wasting, as well as elevated fibroblast growth factor 23 (FGF23).
Patient laboratory testing on presentation
The patient did not have skeletal deformities or a family history of bone disorders, and his bone density readings were in the osteopenia range. He then underwent a PET gallium (68Ga) DOTATATE study to examine for a mesenchymal tumor, which is the usual source of the excess FGF23 production.
Imaging
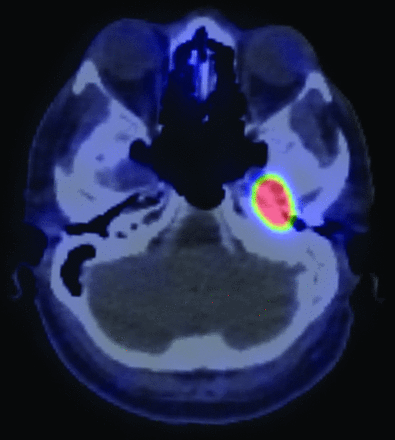
The 68Ga DOTATATE examination demonstrated a focal lesion in the petrous aspect of the left temporal bone (Fig 1), considered highly suspicious for the patient’s culprit lesion. Dedicated imaging of the temporal bone was therefore performed.
68Ga DOTATATE PET/CT demonstrates marked radiotracer uptake within the petrous aspect of the left temporal bone.
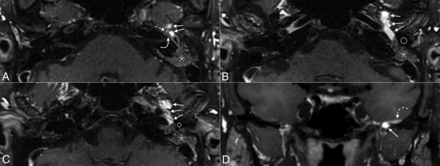
On MR imaging, an avidly enhancing lesion was seen along the cochlear promontory in the middle ear, extending into the anterior hypotympanum along the course of the Eustachian tube (Fig 2). Anteriorly, the mass involved the skull base, encroaching on the foramen spinosum. Posteriorly, the mass closely approximated the geniculate ganglion of the facial nerve. When read in conjunction with the 68Ga DOTATATE examination, this was initially thought to represent a glomus tympanicum tumor with unusual anterior growth. A glomus faciale tumor growing from the geniculate ganglion, phosphaturic mesenchymal tumor (PMT), primary neuroendocrine carcinoma, or metastasis were also considered.
From superior to inferior, axial (A–C) MR images show an avidly enhancing mass (solid arrows) extending from the middle ear along the floor of the middle cranial fossa, with substantial intraosseous involvement. A small portion of the mass (dashed straight arrow) closely approximates the geniculate ganglion of the facial nerve (solid curved arrow) (circles in B and C denote the external auditory canal). The mastoid air cells are completely opacified (asterisks). Coronal image (D) shows the mass extending intracranially, with associated dural thickening (dashed curved arrow).
On a subsequent CT, the tumoral margins were not well-demarcated because the density of the mass matched that of the fluid within the adjacent middle ear and mastoid air cells. However, the CT scans did reveal the mass to be permeative with destruction of the adjacent skull base (Fig 3). In retrospect, the patient did note a long-standing history of left-sided hearing loss and ear fullness without drainage.
Noncontrast temporal bone CT performed 2 days after MR imaging again shows the mass (solid straight arrows) extending from the mesotympanum (A) into the anterior hypotympanum (B), which is widened. The mass is locally destructive (C), causing erosion of the skull base with involvement of the foramen spinosum (dashed straight arrow) and left temporomandibular joint (curved arrow). The margins of the mass are indistinguishable from fluid density within the middle ear and mastoid air cells (asterisks), which remained opacified.
Operative Report
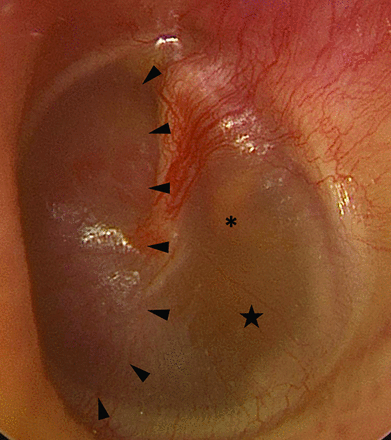
On preoperative otoscopy, the mass was easily visible within the middle ear and less intensely red than a typical paraganglioma (which is typically deep red) (Fig 4). Considering the imaging findings and clinical history, the likely diagnosis was PMT, and surgical treatment was recommended. Tumoral resection was accomplished via a left middle cranial fossa craniotomy. Intraoperatively, the tumor was noted to have eroded through the middle fossa floor just posterior and lateral to foramen spinosum. No involvement of the subtemporal dura was noted intraoperatively, though this may have been due to the dural thickening noted on imaging being reactive in nature rather than tumoral extension. It appeared to originate at the anterior aspect of the Eustachian tube and extended along the Eustachian tube inferiorly into the middle ear. The tumor was quite vascular and required piecemeal excision with frequent use of hemostatic agents and bipolar cautery during resection. Angled instruments and otologic endoscopes were used to release the inferior extent of the tumor from the middle ear. Ultimately, gross total resection was achieved. The patient had an uneventful postoperative course without signs of CSF leak or cranial nerve deficits.
Preoperative otoscopy of the left tympanic membrane reveals a reddish mass (arrowheads) within the anterior mesotympanum. An amber effusion (star) is seen posterior to the mass, surrounding the incudostapedial joint (asterisk).
Pathology
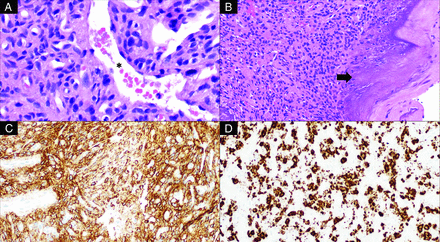
Histologic examination of the tumor revealed a highly vascular neoplasm composed of predominantly spindled tumor cells with bland, ovoid nuclei with interspersed staghorn-like vessels (Fig 5). There were scattered coarse, flocculent calcifications. Neoplastic cells showed overall monotonous nuclei, with low proliferative activity and no tumor necrosis. Immunohistochemical studies revealed tumor cells to be diffusely positive for SSTR2A, while negative for chromogranin and synaptophysin. In addition, there was widespread expression by the tumor cells of FGF23 mRNA by chromogenic in situ hybridization. Overall, these findings supported the final diagnosis of a morphologically benign PMT.
Histologic examination of this tumor reveals bland, spindled-to-stellate neoplastic cells (A, H&E, original magnification ×400) situated in a hyalinized matrix with a well-developed capillary network including ectatic, staghorn vessels (asterisk) as well as characteristic deposition of coarse calcification (B, H&E, original magnification ×200; arrow). The tumor is reactive for SSTR2A (C, immunohistochemistry, original magnification ×200) and shows evidence of FGF23 mRNA expression (D, chromogenic in situ hybridization, original magnification ×200).
Histologically, PMTs are highly vascular and composed of bland, spindled-to-stellate cells in a distinctive myxoid or myxochondroid matrix with flocculent calcification.1⇓-3 FN1-FGFR1 or FN1-FGF1 fusions are common in PMTs (up to approximately 50%), which may activate the FGFR1 pathway leading to increased expression of the FGF23 gene and protein.1⇓-3 FGF23 expression can be detected by reverse-transcription polymerase chain reaction, chromogenic in situ hybridization, or immunohistochemistry.1,2
The histologic differential diagnosis of this tumor includes solitary fibrous tumor, mesenchymal chondrosarcoma, and paraganglioma. Solitary fibrous tumors can have staghorn vessels and have a similar, bland, spindle cell appearance. However, solitary fibrous tumors lack the characteristic matrix and calcifications of PMTs and would not express SSTR2A or FGF23 mRNA.2,3 Mesenchymal chondrosarcomas have malignant, small, round cells; lack the characteristic calcified matrix of a PMT; and would not express FGF23 mRNA. A highly vascular paraganglioma may, at times, be morphologically reminiscent of PMT. However, the immunophenotype of PMT (positive for SSTR2A and FGF23 mRNA, negative for chromogranin and synaptophysin) excluded this possibility. Furthermore, although paragangliomas and PMTs can both exhibit high vascularity, paragangliomas lack the characteristic chondromyxoid matrix and calcifications of PMT. Unlike PMTs, paragangliomas have a characteristic nested (zellballen) architecture composed of neuroendocrine cells and are positive for neuroendocrine markers (eg, synaptophysin and chromogranin). The tumor presented here did not have the classic morphology of paraganglioma and was negative for synaptophysin and chromogranin.
DISCUSSION
PMTs are exceedingly rare tumors that arise primarily from bones and connective tissue. They are typically seen in middle-aged adults without an apparent sex predilection. Pediatric cases have been infrequently reported.4 The median age at the time of diagnosis is 44–48 years of age.5,6 Tumor locality is unpredictable; PMTs can be found in any osseous or soft-tissue location and uncommonly involve the skin.7 More than half of the tumors are found within the extremities, with the femur being the most common site.8 Within the head and neck, the sinonasal region is most common, followed by the mandible and maxilla. Although histologically benign, rare cases of malignant transformation and metastases have been reported.8⇓-10
Clinically, PMTs are associated with tumor-induced osteomalacia (TIO), also known as oncogenic osteomalacia, a paraneoplastic syndrome characterized by widespread reduction of osteoblastic activity. PMTs secrete FGF23, a peptide hormone-like regulator of phosphate levels, which decreases re-absorption of phosphate in the proximal tubules of the kidneys, causing it to be wasted within the urine. The downstream suppression of osteoblasts and mobilization of calcium and phosphate from the bones lead to widespread hypophosphatemic osteomalacia.11 Patients present with bone pain, insufficiency fractures, and gradual-onset muscular weakness.12
A rare subtype of PMT has recently been identified as being nonphosphaturic.13 The descriptions of these tumors are heterogeneous. Some might produce negligible amounts of FGF23 or are thought to exist in patients with a compensatory mechanism in place.3 Some are thought to produce a deformed and/or inactive form of the FGF23 protein.14 Other tumors may simply have been identified in patients before symptomatic osteomalacia had developed.15
Unfortunately, the rarity of PMTs makes them notoriously under-recognized by clinicians. Many patients are symptomatic for years or decades before the tumor is diagnosed.16,17 The diagnosis typically is based on a combination of clinical history, laboratory values, and imaging. Specifically, elevated serum FGF23 levels in a patient with hypophosphatemia and normal renal function is highly suggestive of a PMT. However, TIO has a known association with multiple other tumors, typically of mesenchymal origin, including solitary fibrous tumors, giant cell tumors, chondrosarcomas, and osteosarcomas.18,19 Most, but not all, of these tumors result in TIO through the secretion of FGF23; some secrete various other factors such as frizzled-related protein-4, FGF7, and matrix extracellular phosphoglycoprotein.20
Once the diagnosis of TIO has been established, various nuclear medicine studies can be used to search for the culprit lesion, including 68Ga DOTATATE, technetium Tc99m-octreotide, and FDG PET/CT scans.21 68Ga DOTATATE is now the favored technique, having been shown to have the greatest sensitivity for tumoral detection.15, 22⇓-24 This superiority of 68Ga DOTATATE over other modalities is thought to be due to the higher affinity of its radiotracer to somatostatin receptors 2 and 5, which are expressed by PMTs.23 Nevertheless, the sensitivity of these examinations is suboptimal. A series by El-Maouche et al23 found that 68Ga DOTATATE examinations found a PMT in just more than half of patients with hypophosphatemic TIO, while indium-111 (111In) pentetreotide (OctreoScan) SPECT/CT and [18F] FDG identified <50%.
PMTs are not encapsulated and tend to be locally infiltrative.12 On CT, intraosseous PMTs are osteolytic with a narrow zone of transition and internal matrix; soft-tissue masses are hypodense and enhance.25 On MR imaging, tumors are isointense to soft tissues on T1-weighted imaging and demonstrate enhancement.26 Intralesional T2 signal is variable. Most commonly, tumors are T2-hyperintense, though Broski et al22 noted that PMTs characteristically have small foci of intralesional T2 hypointensity. Some may be partially cystic. Larger tumors may have intralesional flow voids.27 However, most PMTs are small and slow-growing, and tumor localization is highly variable. Thus, they may present a diagnostic conundrum for radiologists, particularly when the diagnosis of TIO is unknown, or, as in this case, if the PMT mimics the appearance of a more common entity.26
The only known definitive treatment for TIO is surgical resection of the tumor. Fortunately, removal of PMTs results in complete resolution of the biochemical and physical sequelae of the paraneoplastic syndrome.7 Because PMTs are often locally invasive, a wide excision is needed to successfully obtain tumor-free margins.28 Although local recurrence may occur, surgical resection is considered curative in approximately 90% of cases.27,29 Cryoablation has been successfully used to treat residual tumor after resection.30 For patients with unresectable tumors, a fully human monoclonal antibody against FGF23, burosumab, has been shown to provide biochemical and symptomatic improvement and was recently FDA-approved.31
Difficult cases are excellent learning opportunities, and this case is no exception. Despite the difficulty of the resection, the surgeons indicated that the available preoperative imaging was excellent, and they stated that additional imaging would not have changed the surgical approach. In retrospect, the differential considerations given in the initial radiology report could have been more precise. The case was challenging due to its location in an anatomic region that can support the growth of paragangliomas. Nevertheless, the clinical history of TIO should have served as a more convincing indicator that the tumor identified on imaging represented a PMT. Incorrectly favoring a paraganglioma over a PMT could have delayed or even prevented surgery because the purpose of the examination was to search for a lesion responsible for the patient’s TIO. Fortunately, the patient ultimately underwent the best treatment course. He is expected to make a full recovery and will undergo routine surveillance imaging to monitor for recurrence.
Case Summary
PMTs are extremely rare entities that are associated with TIO.
The tumors are small and can be found nearly anywhere in the body, making them a common mimic of more common malignancies.
Surgical resection of the tumor results in resolution of a patient’s osteomalacia.
Histologically, PMTs have an unusual, hyalinized matrix that undergoes calcification with frequent expression of FGF23 mRNA.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 3, 2022.
- Accepted after revision March 17, 2022.
- © 2022 by American Journal of Neuroradiology