Abstract
SUMMARY: Current guidelines for primary and secondary prevention of stroke in patients with carotid atherosclerosis are based on the quantification of the degree of stenosis and symptom status. Recent publications have demonstrated that plaque morphology and composition, independent of the degree of stenosis, are important in the risk stratification of carotid atherosclerotic disease. This finding raises the question as to whether current guidelines are adequate or if they should be updated with new evidence, including imaging for plaque phenotyping, risk stratification, and clinical decision-making in addition to the degree of stenosis. To further this discussion, this roadmap consensus article defines the limits of luminal imaging and highlights the current evidence supporting the role of plaque imaging. Furthermore, we identify gaps in current knowledge and suggest steps to generate high-quality evidence, to add relevant information to guidelines currently based on the quantification of stenosis.
ABBREVIATIONS:
- AHA
- American Heart Association
- IPH
- intraplaque hemorrhage
- LRNC
- lipid-rich necrotic core
Acute ischemic stroke is a major cause of morbidity and mortality worldwide, accounting for approximately 5% of disability-adjusted life years and >10% of deaths. Approximately 20% of patients with stroke/TIA have an ipsilateral carotid stenosis of >50%,1,2 and about one-third (about 10% all patients with stroke) had no warning symptoms such as transient ischemic attacks.3
Carotid artery stenosis is a well-established risk factor for ischemic stroke. Determining the best primary and secondary stroke prevention strategies for asymptomatic and symptomatic patients with carotid stenosis is a priority. The current guidelines for the management of both symptomatic and asymptomatic atherosclerosis are based on randomized trials comparing medical therapy with surgical interventions using the degree of stenosis together with symptom status without consideration of plaque morphology and composition. These were published before the advent of vessel wall imaging using MR imaging and high-resolution CT angiography (Figs 1 and 2)
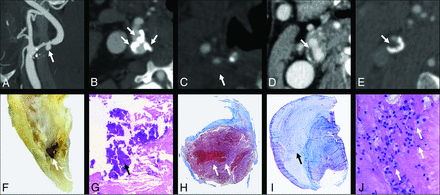
Different CT features. A, Plaque ulceration (arrow) is shown with the corresponding macroscopic specimen (F). B, Multiple coarse calcifications (white arrows) within the plaque are visible with the corresponding example in the H&M histologic view (G, arrow points at a calcification). The IPH is visible in H (white arrows) with the corresponding CTA that shows hypodense plaque in C (Hounsfield unit value = 18; white arrow). I, A stable plaque with a prominent fibrous cap with the major part of the plaque with collagenous connective tissue (black arrow) is shown with the corresponding CT section (D, white arrow). E, The presence of a hypodense plaque (Hounsfield unit = 37) with the corresponding histopathologic slide showing multiple inflammatory cells (J).
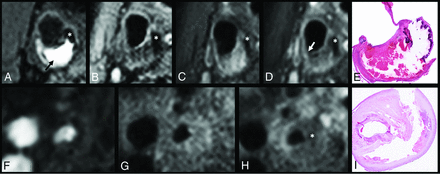
Upper row: Coregistered MPRAGE (A), T2-weighted TSE (B), pre- and postcontrast T1WI TSE MR images (C and D), and a corresponding histologic section (E) of a cross-section of the carotid artery with plaque. A large intraplaque hemorrhage can be recognized as a hyperintense region compared with surrounding muscle tissue in the bulk of the plaque on the MPRAGE image (arrow). Calcification can be identified as a region with hypointense signal on all 4 MR imaging weightings. On the postcontrast T1-weighted TSE image, the region with signal enhancement shows the fibrous cap (between the lumen and intraplaque hemorrhage). The disruption of this enhancement (white arrow) indicates that the fibrous cap is thin or ruptured at this location. Lower row: Coregistered TOF (F), pre- and postcontrast T1-weighted TSE MR images (G and H), and the corresponding histologic section (I) of a cross-section of the carotid artery with a plaque. An LRNC is present in the bulk of the plaque with no or slight contrast enhancement on the postcontrast T1WI (asterisk).
From the first NASCET report that demonstrated the association between high-grade stenosis and outcome, a important evolution in both the surgical approach and medical treatment has occurred. The risk of morbidity and mortality during revascularization procedures, in particular during carotid endarterectomy, has decreased, with a reduction of mortality and severe complications.4⇓-6 Moreover, several trials have provided evidence strengthening conservative medical treatment of carotid disease, including the protective effects of high-dose statin therapy and anti-inflammatory therapy such as the interleukin-1β innate immunity pathway.7⇓⇓-10 Recent meta-analyses provide evidence that atherosclerosis can be reversed (“plaque regression”) with high-dose lipid-lowering therapy,11 and high-dose statins may shift vulnerable plaque from a high lipid content to a more stable calcified plaque.11⇓-13 Imaging of carotid plaque morphology may, therefore, more accurately reflect the pathobiology of the plaque itself, allowing estimation of plaque risk.14 This could lead to a more cost-effective selection of expensive endovascular/surgical management options.9,15
It has been >30 years since the landmark carotid surgery trials defined the degree of carotid stenosis as an important imaging biomarker for surgical management, leading to improved outcomes. However, despite this advance, carotid atherosclerotic disease still accounts for significant morbidity and mortality suggesting the need for a variation in the management and risk stratification of subjects with carotid artery pathology based on the new diagnostic potentialities.
The purpose of this consensus document is to review the current literature, identify new imaging metrics that are associated with future cerebrovascular events and to discuss therapeutic options for specifically targeting these features. Having done so, a roadmap for multicenter diagnostic and therapeutic trials incorporating these imaging biomarkers as inclusion criteria is provided to assess patient outcome compared with management based only on the degree of stenosis.
Summary and Analysis of Existing Guidelines
In this section, we have developed a summary and analysis of existing guidelines. Currently, moderate (50%–69%) and severe (70%–99%) carotid artery stenoses are considered the key parameters together with the symptomatic/asymptomatic status of the patient in deciding management approaches. These are based primarily on NASCET results.16,17 A report published in Stroke, in 2015,18 identified 34 guidelines from 23 different regions/countries in 6 languages, in which 4 scenarios were highlighted:
Asymptomatic patient at average surgical risk with stenosis
Asymptomatic patient at high surgical risk (because of comorbidities, vascular anatomy, or undefined reasons) with stenosis
Symptomatic patient at average surgical risk with stenosis
Symptomatic patient at high surgical risk (because of comorbidities, vascular anatomy, or undefined reasons) with stenosis
In all 4 scenarios, the degree of stenosis (≥ 50%) was the key point considered for treatment. In 33/34 guidelines, treatment was considered only for symptomatic subjects. For symptomatic patients with high carotid endarterectomy risk, medical treatment alone was not endorsed in any guidelines, though the possibility was considered as an alternative option in 2.19⇓-21 Only 1 guideline advised medical treatment alone for patients with asymptomatic carotid stenosis.
The European Society for Vascular Surgery and the European Society of Cardiology developed consensus recommendations for asymptomatic patients, recommending that plaque morphology features be considered.22,23 The only variation compared with the 2011 guidelines was that carotid endarterectomy is indicated in the presence of ≥1 imaging characteristic that may be associated with high stroke risk in asymptomatic subjects. These data indicate that the guidelines currently used worldwide do not consider the imaging-based plaque morphology/composition as a parameter for the therapeutic option and that the class of risk is based on the mere degree of stenosis and symptomatic/asymptomatic status of the patient.
However, in past years, landmark articles showing the impact of imaging-based features of carotid artery plaque vulnerability in symptomatic and asymptomatic patients with mild stenosis24 and the benefit of conservative medical treatment for the plaque stabilization and reversion have been published,7,9,13 highlighting the need for changes in the forthcoming guidelines.
Evidence That Imaging of Plaque Composition Predicts Ischemic Stroke Risk
In the past years, evidence has accumulated in pathology and imaging fields demonstrating that plaque composition plays a key role in the vulnerability of the carotid artery plaque.
Histopathology of Unstable Plaque.
Coronary atherothrombosis was described >150 years ago, and carotid stenosis was coupled with the pathophysiology of ischemic stroke >70 years ago. The associations between histopathologic features and increased risk of stroke were described in the 1970s and 1980s, noting the association between fibrous cap rupture and thromboembolism (Fig 1)25⇓-27 and identification of intraplaque hemorrhage (IPH) as a marker of recent symptom-producing plaques.28⇓-30
Histopathologic studies showed that vulnerable plaques were characterized by a thin or ruptured fibrous cap, endothelial erosions, enhanced inflammation, large lipid-rich necrotic cores, immature intraplaque neovascularity, and IPH, whereas stable and asymptomatic lesions typically contain more fibrous tissue and more calcification.31,32
However, the authors found that ulceration, IPH, and organizing or organized thrombi were also found in both symptomatic and asymptomatic stenotic plaques examined pathologically,29,33⇓⇓⇓⇓⇓⇓-40 suggesting a complex pathobiologic scenario for the plaque rupture. The authors found that biologic variability in plaque morphology also plays a role.
Fisher and Ojemann41 noted that “the variations in the microscopic appearance of the plaque contents seemed to be unending.” The authors found that the position of the lipid/necrotic core and thinning of the cap may be the most significant features predisposing to plaque rupture.27,40 Most interesting, decreasing fibrous cap thickness increases the circumferential stress on a plaque, whereas increasing stenosis severity decreases circumferential stress.42 This finding may help to explain why stroke risk tends to be lower in patients with critical stenosis compared with high-grade stenosis.
The American Heart Association (AHA), in 1995, published43 a detailed classification scheme designed to be used as a histologic template for images obtained by a variety of invasive and noninvasive techniques in the clinical setting. In the AHA scheme (Table), revised in 2000,44 the lesions are designated by Roman numerals, which indicate the usual sequence of lesion progression from the initial lesion, type I to type VIII, in which the fibrous tissue changes within the plaque predominate. This classification was in MR imaging and CT studies (Table).45,46 Virmani et al47 built on the Stary system to more closely focus on erosion, rupture, and thinning of the fibrous cap, increasingly prevalent in the population due to widespread use of statins. The result of these developments in plaque phenotyping have converged into the most widely accepted system in use today,48 which also suggests that further development will be possible once modalities to recognize the lesion by noninvasive means are addressed in this roadmap.
AHA classification and AHA-MR imaging–based classification
Plaque Vulnerability in Patients with Mild or No Stenosis.
Conventional angiography tends to underestimate the extent of disease because the lumen can be maintained through positive remodeling of the vessel wall, further exaggerated by the anatomy of the carotid bulb. Patients with lesser degrees of stenosis represent a significant proportion of patients with stroke. In the NASCET trial, >40% of those with stroke on follow-up were from the <50% stenosis group.16 Mild stenoses, albeit associated with reduced risk of producing ischemic events, are much more common than severe stenoses and, thus, are associated with a substantial number of events: The estimated prevalence of carotid stenosis of ≥50% in the general population ranges from 2% to 8% and the estimated prevalence of stenosis of ≥80% ranges from 1% to 2%.49 Detection of high-risk lesions in ever decreasing degrees of carotid stenosis will potentially require either higher resolution imaging or more conspicuous imaging biomarkers.
Features of plaque vulnerability are related to the occurrence of ischemic events independent of the degree of stenosis: Studying plaques with lower levels of luminal stenosis separates the effects of hemodynamic compromise caused by the luminal narrowing and vessel wall pathology on clinical outcomes. In a group of patients studied recently presenting with imaging-proved acute stroke with no significant stenosis (<50%), up to half were shown to have IPH in the carotid artery ipsilateral to the stroke, suggesting a possible source of cerebral50,51 emboli. Some morphologic features, such as ulceration, are also associated with the occurrence of ischemic events independent of the degree of stenosis.52 In a meta-analysis of 8 studies including 689 patients, the presence of IPH at baseline was associated with a 6-fold higher risk of cerebrovascular events, with an annualized event rate of 17.7% compared with 2.43% in patients with no IPH.53 In a separate meta-analysis of 9 studies and 779 subjects, the hazard ratios for subsequent stroke/TIA were 4.59 for IPH, 3.00 for lipid-rich necrotic core (LRNC), and 5.93 for thin/ruptured fibrous cap.54 Last, another meta-analysis recently published in 2019, including 560 patients with symptomatic and 136 patients with asymptomatic carotid stenosis, reported that the presence of IPH at baseline increased the risk of ipsilateral stroke both in symptomatic (hazard ratio = 10.2; 95% CI, 4.6–22.5) and asymptomatic (hazard ratio = 7.9; 95% CI, 1.3–47.6) patients. Among patients with symptomatic carotid stenosis, annualized event rates of ipsilateral stroke in those with IPH versus those without IPH were 9.0% versus 0.7% (<50% stenosis), 18.1% versus 2.1% (50%–69% stenosis), and 29.3% versus 1.5% (70%–99% stenosis). Multivariate analysis identified IPH (hazard ratio = 11.0; 95% CI, 4.8–25.1) and a severe degree of stenosis (hazard ratio = 3.3; 95% CI, 1.4–7.8) as independent predictors of ipsilateral stroke.55
Plaque with Severe Stenosis and a Low Likelihood of Rupture.
Several studies have demonstrated that plaque calcification is a stabilizing factor in carotid artery stenosis and is more common in asymptomatic than in symptomatic plaques.56 Histopathologic studies demonstrated that plaques with a high burden of calcification have lower rates of inflammation, macrophage burden, neovascularization, and IPH, lending further support to the use of plaque imaging as a risk-stratification tool.14
Plaque Progression and Regression
With improvements in MR imaging, sonography, and CT, it is now possible to directly visualize the carotid wall volume and plaque composition as the vessel wall disease evolves from early/mild atherosclerosis to late-stage/severe-stage atherosclerosis.57 Progression of plaque morphology with increasing vessel wall volume or progression of plaque components with increasing size of vulnerable plaque features or both are associated with an increased risk of future cerebrovascular and cardiovascular events.58 Furthermore, direct visualization of the plaque response to medical therapy offers the potential for individualization of atherosclerosis treatment.59 To use imaging for assessing the response of carotid plaque to drug therapy, one needs to determine the reproducibility of the imaging.
Evidence of Plaque Progression and Regression.
In a prospective, case-controlled study of asymptomatic patients with moderate carotid stenosis, LRNC size increased in plaques with IPH compared with plaques with no IPH.60 The role of IPH-induced plaque progression was demonstrated in a later study of mildly stenotic asymptomatic patients in whom IPH was found to significantly increase plaque size.61 This finding suggests that IPH may occur before stenosis becomes severe and may drive the stenotic phenotype.59 In a prospective study of asymptomatic patients with moderate stenosis, the LRNC size governed the risk of future surface disruption, suggesting that urgent lipid-lowering therapy to prevent the transition from stable to unstable atherosclerotic disease may be warranted.62
Plaque progression is a major risk factor for the development of future ischemic events. Longitudinal studies have demonstrated that the presence of plaque hemorrhage63 (as determined by MR imaging) or a hypoechoic plaque (on sonography) is a major risk factor for plaque progression.64 Another mechanism for rapid significant progression of plaque volume is silent plaque rupture and healing.65
With regard to evidence of plaque regression, lipid-lowering treatment, predominately with statin therapy, has been shown to decrease carotid plaque size and composition. Corti et al66 were the first to show a decrease in vessel wall thickness and vessel wall area. Observational studies have all used the 1-year timeframe to study changes in vessel wall size.59 Because wall volume showed a greater reduction in more diseased segments with statin therapy,67 it seems that carotid MR imaging is best suited to yearly follow-up of patients with known carotid stenosis. Studies have shown that statin therapy is associated with a decrease in LRNC and an increase in fibrous tissue,68 which precedes any reduction in plaque volume.67 Information from natural history studies suggests that IPH may override the beneficial effects of statin therapy, but the statin type and dose were not randomized or uniform.61 No prospective trials exist testing the hypothesis that the deleterious effects of IPH can be modified with very intensive lipid-lowering therapy.
Underhill and Yuan59 summarized the use of MR imaging monitoring of carotid plaque in clinical trials, noting the following: 1) The rate of change is slower in plaques with <50% LRNC volume, though improved image quality may allow detection of change at 6 months, 2) changes in plaque composition precede changes in plaque morphology, and 3) LRNC at baseline is needed to monitor treatment effect with MR imaging.
Sonography provides a sensitive measure of carotid plaque regression. Carotid plaques are focal and progress along the artery wall 2.4 times faster than they thicken.69 Spence and Hackam70 reported their experience in 4387 patients imaged with serial carotid total plaque area. In this cohort, they intensified medical therapy for patients with documented plaque progression despite guideline-based medical therapy. By “treating arteries instead of risk factors,” they significantly decreased the incidence of plaque progression and cardiovascular events, and microemboli on transcranial Doppler sonography markedly declined with intensification of medical therapy.71
Updated Drug Therapy: Evidence and Impact
It is demonstrated that a group of potentially modifiable risk factors (hypertension, regular physical activity, dyslipidemia, diet, obesity, psychosocial factors, smoking, cardiac causes, alcohol consumption, and diabetes mellitus) account for 90% of the population-attributable risks of stroke,72 and some classes of drugs can significantly impact these factors. In this section we will explore the latest evidence in the use of imaging and its impact on the drug therapy in the prevention of stroke.
Lipid-Lowering Therapy.
Studies have shown that treatment with statins reduces the risk of stroke in patients at high risk for atherosclerosis by 21% and that this risk reduction has been associated with each 1-mmol/L (39 mg/dL) decrease in low-density lipoprotein.73,74 In a meta-analysis by Cannon et al,75 published in 2006, high-intensity statin treatment reduced nonfatal cardiovascular events and led to lower stroke incidence, even in healthy individuals, with low-density lipoprotein levels of <130 mg/dL and high-sensitivity C-reactive protein levels of >2 mg/L76 Two randomized controlled trials have shown improved cardiovascular diseases outcomes with the addition of nonstatin lipid-lowering medications: ezetimibe77 and evocolumab.78 The proprotein convertase subtilisin/kexin type 9 inhibitors achieve very low nonstatin low-density lipoprotein thresholds.79 A study published in 2016 showed that the duration of statin therapy is associated with the regression of carotid plaque neovasculature measured with dynamic contrast-enhanced MR imaging,80 and these results were confirmed by another group in 2019,81 again with dynamic contrast-enhanced MR imaging, which demonstrated that statins rapidly and significantly decreased adventitial and plaque neovascularization at 3 months.
Antiplatelet Therapy.
In a study published in 1997,82 the introduction of aspirin within 48 hours after ischemic stroke led to a significant reduction in recurrence within 2 weeks,83 and the addition of dipyridamole and clopidogrel added significant benefit to secondary stroke prevention.84⇓⇓-87 Evidence suggests that while benefit occurs within 48 hours of starting aspirin for stroke prevention, there is no further benefit after 2 months.88 The benefits of long-term treatment with dual antiplatelet therapy (aspirin plus clopidogrel) in patients with acute coronary syndrome were never replicated in patients with stroke and are associated with more bleeding complications.53,62,89 The impact of antiplatelet therapy on carotid artery plaque has been explored: in 2019, a sonography-based trial was published that explored the efficacy and usefulness of an antiplatelet drug (cilostazol) on the progression of carotid intima-media thickness, and the authors found that it may inhibit plaque formation.90
Anticoagulation Therapy.
In 2017, Eikelboom et al91 published the results of the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial. In this study, patients with coronary, peripheral, and carotid artery disease (symptomatic or asymptomatic) were included, and a combination of an anticoagulant (2.5 mg of rivaroxaban twice a day) and aspirin proved superior to aspirin alone and 5 mg of rivaroxaban twice a day. The outcome of ischemic and hemorrhagic events was significantly in favor of patients in the combined treatment group, and efficacy outcomes were mainly driven by a 50% relative-risk reduction in ischemic stroke risk (P < .001). The recently published Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients with Recent Embolic Stroke of Undetermined Source (NAVIGATE ESUS) trial, performed in 4723 participants with available intracranial CTA or MRA, showed that among participants with evidence of systemic atherosclerosis by either history or imaging (n = 3820), recurrent ischemic stroke rates were similar among those assigned to rivaroxaban (5.5%/year) versus aspirin (4.9%/year) (hazard ratio = 1.1; 95% CI, 0.84–1.5).92
Anti-Inflammatory Therapy.
Atherosclerosis is considered a predominantly a lipid-driven, chronic, low-grade inflammatory disease of the arterial wall.93 Anti-inflammatory strategies are increasingly being considered as an attractive strategy to further reduce the residual risk of atherosclerotic cardiovascular disease.94 The administration of canakinumab (a monoclonal antibody against interleukin-1β) reduces the incidence of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death. Colchicine is another anti-inflammatory drug that may result in plaque stabilization,95 reducing the incidence of noncardioembolic ischemic stroke in patients with stable coronary artery disease.96 Promising imaging-based studies have shown the impact of anti-inflammatory therapy on plaque progression and composition in the coronary arteries,97 suggesting similar effects and ability to assess them in the carotid arteries as addressed by this roadmap.
Suggested Roadmap
There are 2 related-but-distinct clinical tasks, phenotype classification to categorize patients to their individual disease mechanism to identify the treatments to which they would most likely respond, and risk stratification to identify how urgent interventional treatments may be, to allow patients to benefit from tailored therapeutics to ultimately lower or reverse disease progress and ultimately shift the at-risk population to less acute manifestations of disease.
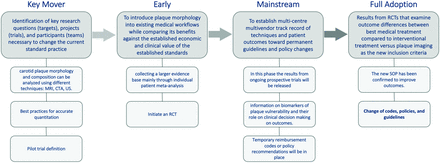
Sufficient data already exist to formally incorporate some plaque imaging in the management of atherosclerotic carotid artery disease. It is becoming increasingly important to begin the development of a common roadmap for changing the current standard of practice. We propose a 4-phase roadmap (Fig 3). This section outlines the broad overview of the roadmap, and more details are given in the Online Supplemental Data.
Roadmap graphic flow chart showing the 4 phases: key mover, early, mainstream, and full adoption. The lighter gray boxes represent the components of the various stages of the roadmap. RCT indicates randomized controlled trial; SOP, standard of practice; US, ultrasound.
Key Mover (The Phase in Which the Field Has Been).
Identification of key research questions (targets), projects (trials), and participants (teams) necessary to change the current standard of practice is currently confined to considering the degree of stenosis and the symptom status of the patients. This change begins by examination of existing evidence that is valuable for identifying existing knowledge gaps through systematic reviews. The results of these reviews can then be used to formulate key research priorities for guiding the development of randomized controlled trials. Outcomes from such randomized controlled trials can then be used to initiate policy discussions, including clinical implementation recommendations and the development of new reimbursement codes as required.
Early (The Phase the Field Is Entering).
In this early phase, one introduces plaque morphology into existing medical workflows while comparing its benefits against the established economic and clinical values of the established standards. One must start the development of local reimbursement codes/policies in readiness for a larger body of evidence of efficacy and patient benefit. Among the most important activities of this phase is to transition beyond retrospective studies to prospective ones. The retrospective studies are inherently limited due to the fundamental confounding of the current standard of care with the incidence of events; the ability to study positive benefits of plaque morphology assessment as to the improvement in patient outcomes can only be properly studied in 2-arm studies that allow study of the hypothesized improvement without being hampered by data collection that, by definition, is not allowed to use it. The data from these studies are expected to develop a better tool for determining the best treatment option for atherosclerosis and inform a better standard of care to reduce the incidence of adverse neurologic symptomatology and poor outcome (eg, ischemic stroke) for patients with known or suspected carotid artery disease.
Mainstream.
To reach the mainstream stage, one must address the economic impact, and indications of different organizations should be taken into account to identify an optimal balance in terms of diagnostic stratification of the risk and economic impact of the process. One must establish a multicenter, multivendor track record of techniques and patient outcomes toward permanent guidelines and policy changes. A collaborative and central data base construction for rapid, large data collection and analysis would accelerate this process. Standardized imaging protocols would allow accrual from both clinical (eligible retrospective and prospective) and ongoing research imaging, with capture of standardized patient clinical data ideally with follow-up, requiring appropriate patient consent.98
Full Adoption.
Results from randomized controlled trials that examine outcome differences between the best medical treatment compared with interventional treatment (carotid endarterectomy) with treatment selection randomized to the current standards (degree of stenosis) versus plaque imaging as the new inclusion criteria will be adopted. Change in clinical practice would lead to an update of policies, guidelines, and billing codes. In parallel with the stages as they effect treatment of patients with signs and symptoms, there is an even broader application in population-based screening. Whereas the US Preventive Services Task Force has presently recommended against screening,99 the nature of these assessments is to await the development of more powerful technologies and/or the evolution of disease prevalence until such capability is considered to have reached a crossover point. No doubt the stages that we have identified will provide additional input to this process. Regardless of whether population-based screening does or does not reach the point of being recommended, our roadmap will meet the needs of the patients with signs and symptoms regardless and, in so doing, increasingly provide screening options for patient subpopulations that would also benefit.
CONCLUSIONS
In this roadmap consensus article, we have defined the limits of luminal imaging and highlight current evidence supporting the role of plaque imaging in risk stratification and treatment of carotid artery atherosclerosis and stroke. These recommendations are supported by evidence that highlights the limits of risk stratification based on the degree of luminal stenosis alone and emphasize the predictive power of other features such as the presence of IPH. Outcome trials, which confirm image-based information and can act as a primary parameter for choosing therapeutic interventions and predicting outcomes, are fundamental for the full adoption of a plaque-imaging-based approach. This body of evidence needs to be merged with evidence from trials that show the effects of pharmaceutical agents to better understand the overall benefits of incorporating plaque imaging metrics. This roadmap details the process for acquiring the necessary high-quality evidence to support the incorporation of plaque imaging in risk stratification and the management of carotid artery atherosclerotic disease.
Acknowledgments
The authors would like to express their deepest gratitude to Riccardo Cau, Alessandra Serra, Mueez Aizaz, and Mohamed Kassem for their support and assistance with this project
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US Government. The identification of specific products or scientific instrumentation does not constitute endorsement or implied endorsement on the part of the author, Department of Defense, or any component agency.
Disclosures: Mauricio Castillo—UNRELATED: Employment: University of North Carolina. Peter Rothwell—UNRELATED: Board Membership: ARRIVE Trial Executive Committee; Consultancy: BMS Axiomatic Trial Data and Safety Monitoring Board; Payment for Lectures Including Service on Speakers Bureaus: Abbott for lecture on Patent Forame Ovale closure; Other: lecture on TIA for AstraZeneca. Max Wintermark—UNRELATED: Consultancy: Magnetic Insight, Subtle Medical, EMTensor, Icometrix, Nous Infosystems. Chun Yuan—UNRELATED: Grants/Grants Pending: National Institutes of Health, American Heart Association, Philips Healthcare.* Andrew Buckler—UNRELATED: Employment: Elucid Bioimaging. Davide Capodanno—UNRELATED: Board Membership: Medtronic, Comments: speaker’s fee; Consultancy: BIOTRONIK, Comments: speaker’s fee; Employment: Boston Scientific, Comments: speaker’s fee; Expert Testimony: Daiichi Sankyo, Comments: speaker’s fee; Grants/Grants Pending: Boehringer Ingelheim, Comments: speaker’s fee; Payment for Lectures Including Service on Speakers Bureaus: Bayer AG, Comments: speaker’s fee; Payment for Manuscript Preparation: AstraZeneca, Comments: speaker’s fee. Ulf Hedin—UNRELATED: Employment: Karolinska Hospital; Grants/Grants Pending: Swedish Research Council. Waleed Brinjikji—UNRELATED: Consultancy: MicroVention, Cerenovus*; Stock/Stock Options: Marblehead Medical LLC. Thomas Hatsukami—UNRELATED: Grants/Grants Pending: investigator-initiated grant from Philips Healthcare, completed December 31, 2018.* Christopher Hess—UNRELATED: Personal Fees: GE Healthcare, Focused Ultrasound Foundation, uniQure, Comments: consultant for GE Healthcare and Data and Safety Monitoring Board member for Focused Ultrasound Foundation and uniQure; Nonfinancial Support: Siemens, Comments: research travel. Bruce A. Wasserman—UNRELATED: Grants/Grants Pending: National Institutes of Health R01.* Joanna Wardlaw—RELATED: Grant: National Institute for Health Research Health Technology Assessment Panel, Comments: funded the research.* Ajay Gupta—UNRELATED: Consultancy: ERT; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Siemens, GE Healthcare. Jie Sun—UNRELATED: Grants/Grants Pending: American Heart Association, Institute of Translational Health Sciences.* Niranjan Balu—UNRELATED: Patents (Planned, Pending or Issued): I hold a US patent 9,557,396 but no payment/royalties. *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- Received November 18, 2020.
- Accepted after revision January 26, 2021.
- © 2021 by American Journal of Neuroradiology