Abstract
BACKGROUND AND PURPOSE: Retinal diffusion restrictions were recently identified as a regular finding in acute central retinal artery occlusion. We sought to investigate the influence of technical MR imaging and clinical parameters on the detection rate of retinal diffusion restrictions on standard brain DWI.
MATERIALS AND METHODS: In this retrospective cohort study, MR imaging scans of patients with central retinal artery occlusion were assessed by 2 readers for retinal diffusion restrictions on DWI performed within 2 weeks after vision loss. The influence of clinical and technical MR imaging parameters and the time interval between symptom onset and DWI on the presence of retinal diffusion restrictions were evaluated.
RESULTS: One hundred twenty-seven patients (mean age, 69.6 [SD 13.9] years; 59 women) and 131 DWI scans were included. Overall, the MR imaging sensitivity of retinal diffusion restrictions in acute central retinal artery occlusion was 62.6%–67.2%. Interrater and intrarater agreement for retinal diffusion restrictions was “substantial” with κinter = 0.70 (95% CI, 0.57–0.83) and κintra = 0.75 (95% CI, 0.63–0.88). Detection of retinal diffusion restrictions did not differ with differences in field strengths (1.5 versus 3T, P = .35) or sequence type (P = .22). Retinal diffusion restrictions were consistently identified within the first week with a peak sensitivity of 79% in DWI performed within 24 hours after symptom onset. Sensitivity of retinal diffusion restrictions declined in the second week (10.0%, P < .001). Absence of retinal diffusion restrictions was more prevalent in patients without fundoscopic retinal edema (60% versus 27.1%, P = .004) and with restitution of visual acuity at discharge (75% versus 28.4%, P = .006).
CONCLUSIONS: Retinal diffusion restrictions in acute central retinal artery occlusion can be reliably identified on DWI performed within 24 hours and 1 week after onset of visual impairment. Detectability of retinal diffusion restrictions is dependent on the clinical course of the disease.
ABBREVIATIONS:
- CRAO
- central retinal artery occlusion
- logMAR
- Logarithm of the Minimum Angle of Resolution
- RDR
- retinal diffusion restrictions
- VA
- visual acuity
Sudden and painless monocular visual impairment is the characteristic clinical feature of nonarteritic acute central retinal artery occlusion (CRAO), which is mainly caused by proximal embolism originating from the heart or atherosclerotic lesions of the aortic arch and carotid arteries.1 Patients with CRAO develop persistent and debilitating central scotoma if the blood supply to the eye is not re-established in time. While there is disagreement on the choice of treatment to achieve retinal reperfusion,2 there is consensus to treat as early as possible because the chance for visual improvement dwindles with the duration of retinal ischemia.3
Because macular edema or alteration of retinal arteries may not always be apparent at the first fundoscopic evaluation,4 supplementary diagnostics such as fluorescein angiography and optical coherence tomography are necessary.5,6 Patients with CRAO have an elevated risk of consecutive stroke, which underlines the importance of timely and accurate diagnosis.7,8 One-quarter of patients with acute CRAO show concurrent cerebral infarction on DWI.9⇓-11
We recently identified retinal diffusion restrictions (RDR) as a frequent finding in patients with CRAO on standard 1.5T and 3T brain stroke DWI,12 which contribute to diagnosis of retinal ischemia. While these RDR were accurate in discerning patients with CRAO from those with ischemic stroke and transient ischemic attack, sensitivity for RDR in CRAO was only moderate (up to 0.75).12 To the best of our knowledge, apart from incidental descriptions in case reports or small case series,13⇓-15 no further studies of RDR in CRAO exist as of today. Because available literature is scarce, no information exists on how clinical and ophthalmologic features of CRAO or technical MR imaging parameters contribute to the detection rate of RDR in retinal ischemia. Moreover, the time course of RDR in CRAO is unknown. Therefore, we performed a large retrospective cohort study to further investigate these questions.
MATERIALS AND METHODS
Patients
All consecutive patients with CRAO treated in our institution between January 2010 and December 2019 with available brain MR imaging including diffusion-weighted imaging performed within 2 weeks after clinical onset were included into this single-center retrospective cohort study. Potential candidates were identified through a medical data base inquiry based on the respective codes of the International Classification of Diseases (H34.0-H34.2; H34.8; and H34.9) and the German Operation and Procedure Classification System (3-800 and 3-820).
Patients were included in the study if they met the CRAO diagnostic criteria of sudden, painless, and persistent monocular vision loss accompanied by characteristic fundoscopic features, including retinal opacity, a cherry-red spot, the presence of emboli, attenuation of arteries, and/or optic disc pallor/edema. Patients missing typical ophthalmoscopic findings of CRAO were included if the diagnosis could be based on optical coherence tomography and/or fluorescein angiography. Patients with sole retinal branch occlusion and amaurosis fugax were not included in this study. Furthermore, we did not include patients with a possible diagnosis of giant cell arteritis according to the American College of Rheumatology 1990 criteria.
We systematically recorded relevant patient data, including visual acuities (VA), fundoscopic features, medical histories (including hypertension, hypercholesterolemia, diabetes mellitus, atrial fibrillation, and smoking habits), laboratory findings (including glycated hemoglobin and lipid profiles), evidence of VA restoration at discharge, and intravenous thrombolytic therapy if received. Patients were eligible for rtPA treatment until 4.5 hours after symptom onset. Contraindications for intravenous thrombolysis with rtPA were congruent with those established for the treatment of acute ischemic stroke.16 Categorization of visual impairment at initial presentation was adapted from the World Health Organization International Statistical Classification of Diseases and Related Health Problems (10th revision, 2016):
Category 0: mild or no visual impairment: VA ≥ 0.3 (≤0.52 Logarithm of the Minimum Angle of Resolution [logMAR]).
Category 1: moderate visual impairment: VA < 0.3/≥0.1 (>0.52/≤1.0 logMAR).
Category 2: severe visual impairment: VA < 0.1/≥0.05 (>1.0/≤ 1.3 logMAR).
Category 3: blindness: VA < 0.05 (>1.3 logMAR).
DWI Analysis
MR imaging was performed as part of the routine clinical work-up of patients with CRAO to identify concurrent ischemic stroke on two 1.5T scanners (Magnetom Aera; Siemens) each with 20-channel head coils and a 3T scanner (Magnetom Skyra; Siemens) with a 20-channel head coil. The DTI sequence used for DWI calculation was acquired on a 3T scanner (Magnetom Trio; Siemens) with a 32-channel head coil. TRACE DWI (b = 1000 s/mm2) images from EPI-DWI sequences or calculated from EPI-DTI sequences were evaluated. Section thicknesses were 2.5 mm (DTI), and 3, 5, and 7 mm (DWI). Further sequence details are given in the Online Supplemental Data. DWI was evaluated for RDR by a board-certified neuroradiologist (reader 1, with >15 years of experience in MR stroke imaging), and a radiology resident-in-training for neuroradiology (reader 2, with 2 years of neuroradiologic experience) who were blinded to the CRAO side and clinical data. DWI was considered positive for RDR if a clearly discernable abnormal signal increase was present in the inner wall of the affected globe on at least 2 adjacent slices compared with the contralateral eye. In cases of RDR, reader 1 additionally evaluated the presence of visually correlating low signal on the ADC map as well as concurrent restricted diffusion of the optic nerve. Furthermore, reader 1 performed a second complete DWI review for the presence of RDR 12 months after the first evaluation. The time span between symptom onset and DWI was recorded and categorized into 5 subgroups: ≤24 hours, >24–72 hours, >72 hours to 7 days, >7–14 days, and “unclear” in cases in which definite classification was not possible.
Statistical Analysis
SPSS Statistics for Windows, software (Version 25.0; IBM) was used for statistical analysis. Interrater and intrarater agreement were analyzed using Cohen’s kappa statistics. For this purpose, the observed percentage of agreement Pr(a) and expected Pr(e) were used to calculate the unweighted κ using the formula:
The interpretation of agreement for κ was categorized as poor (κ < 0.00), slight (0.00 ≤ κ ≤ 0.20), fair (0.21 ≤ κ ≤ 0.40), moderate (0.41 ≤ κ ≤ 0.60), substantial (0.61 ≤ κ ≤ 0.80), or almost perfect (0.81 ≤ κ ≤ 1.00), respectively.
We evaluated the impact of different clinical and technical MR imaging parameters for the presence/absence of RDR in CRAO using χ2 statistics and Bonferroni post hoc analysis if applicable. A P value < .05 was considered statistically significant. Descriptive statistics are presented as mean (SD). The aforementioned clinical parameters included the following: visual acuity at presentation, fundoscopic presence of retinal edema, time span between symptom onset and DWI, thrombolytic therapy, and evidence of VA restitution at discharge. Technical MR imaging parameters included electromagnetic field strength and image section thickness.
Study approval was obtained from the local ethics committee (Charité, Universitätsmedizin Berlin, EA1/177/19).
RESULTS
Of 355 candidates identified through our medical data base inquiry, 131 patients matched CRAO diagnostic criteria and had diffusion-weighted imaging performed within 2 weeks after clinical onset. In 4 subjects, artifacts rendered the assessment of the retina on DWI impossible. Consequently, 127 patients (mean age, 69.6 [SD, 13.9] years; 59 women) were included in this study. Table 1 details clinical, fundoscopic, and radiologic characteristics of patients with CRAO.
Clinical, fundoscopic, and radiologic characteristics of patients with CRAO
Prevalence of Retinal Diffusion Restrictions
In total, 131 MR images were analyzed (in 127 patients). RDR was present in 88 (reader 1, 67.2%) and 82 (reader 2, 62.6%) of 131 scans, respectively, with 57 cases (reader 2, 64.8%) showing visually correlating low signal on the ADC map. Both interrater and intrarater agreement for RDR were “substantial” with unweighted κinter = 0.70 (95% CI, 0.57–0.83) and κintra = 0.75 (95% CI, 0.63–0.88). Concurrent restricted diffusion of the optic nerve was noted in 17 cases (reader 1, 13.0%). Figures 1 and 2 illustrate characteristic examples of RDR on 1.5T and 3T DWI.
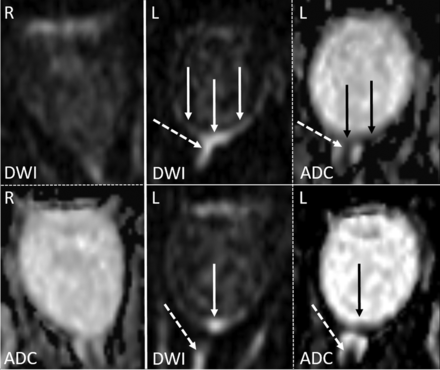
An example of RDR on 1.5T DWI in acute left-sided CRAO (solid arrows) visible on 2 adjacent slices, as well as diffusion restriction of the anterior optic nerve (dotted arrows), all with qualitative ADC reduction. The normal right eye is shown for comparison.
Examples of RDR on 3T diffusion-weighted MR imaging in acute CRAO (DWI, b=1000s/mm2, and ADC images). Left column: In this patient with left-sided CRAO, clear RDR with subtle retinal thickening is shown (white arrows), more pronounced temporally, on 3 consecutive slices (TRACE DWI calculated from the DTI-EPI sequence) and with corresponding qualitative ADC reduction (black arrows). Right column: Two cases of right-sided CRAO are shown with RDR (DWI hypersignal and corresponding visually qualitative ADC reduction). While the upper case is more temporally pronounced, the lower case is more uniformly affecting both the temporal and nasal parts of the retina.
The overall side of distribution of CRAO was balanced with 64 (50.4%) left- and 63 (49.6%) right-sided occlusions. In 1 (reader 1, 0.8%) and 2 cases (reader 2, 1.5%), RDR were falsely attributed to the healthy eye (DWI restriction rated as “absent” for the affected eye).
Association between Retinal Diffusion Restrictions and Clinical Features
Visual acuity on initial presentation was <0.05 (>1.3 logMAR, legal blindness) for most patients (104, 81.9%). Visual impairment of the remaining patients was severe in 10 (7.9%), moderate in 9 (7.1%), and mild in 4 cases (3.2%). There were no statistically relevant differences in the frequency of RDR among the visual impairment groups (P = .07), though RDR were more frequent in patients with VA <0.05 (“blindness”: 75/104 or 72.1%; compared with severe visual impairment: 4/10 or 60%; moderate visual impairment: 5/9 or 55.6%; and mild visual impairment: 2/4 or 50%). Most interesting, the absence of RDR was significantly more frequent in patients with complete restitution of visual acuity at discharge (6/8 or 75%) compared with patients without reported remission (33/116 or 28.4%, P = .006).
Fundoscopic findings are detailed in Table 1. Patients showing neither retinal opacity nor a cherry-red spot during ophthalmoscopic evaluation were significantly more likely to show no RDR (12/20 or 60%) compared with patients with visible retinal opacity and/or cherry-red spot (29/107 or 27.1%, P = .004). No statistical relationship was observed between the presence of attenuated arteries, optic disc pallor/edema, or visible emboli and the presence of RDR on DWI, respectively (data not shown).
Intravenous rtPA was administered in a total of 22 patients with CRAO (17.32%). χ2 testing, however, revealed no differences in the presence of RDR between rtPA-treated and untreated patients with CRAO (P = .58). Likewise, the frequency of RDR did not differ between patients with and without acute cerebral ischemia on DWI (26/36 or 72.2% versus 59/91 or 64.8%, P = .43).
Impact of MR Imaging Parameters on the Detection of Retinal Diffusion Restrictions
Table 2 details the distribution of RDR according to technical MR imaging parameters. MR imaging sensitivity for CRAO detection did not differ significantly regarding differences in field strengths (1.5T versus 3T, P = .14) or sequence types used (3-mm DWI-EPI TRACE versus 2.5-mm calculated DWI-TRACE from DTI-EPI; P = .22). Five- and 7-mm DWI-EPI TRACE was not included in the analysis because of the low absolute number of scans performed (n = 3).
Distribution of retinal DWI restrictions according to MR imaging parameters
Time Course of Retinal DWI Restrictions
Time intervals between clinical onset of visual impairment and DWI were as follows: ≤24 hours in 28 (21.4%), >24–72 hours in 63 (48.1%), >72 hours to 7 days in 23 (17.6%), and >7–14 days in 10 (7.6%) cases. For 7 scans (5.3%), an unambiguous assignment to a time group was not possible. Overall detection rates of RDR were comparable for both readers among time interval groups up to 1 week between clinical onset and DWI. A multiple-comparison χ2 test with post hoc Bonferroni correction identified RDR to be significantly less frequent in DWI performed 1 week after onset of vision loss (>7–14 days, 1/10 or 10.0%; reader 1: P < .001, reader 2: P = .005). Notably, sensitivity for RDR was the highest in DWI performed within 24 hours after CRAO onset (0.79, reader 1), though this difference did not reach statistical relevance. Figure 3 details the distribution and sensitivity of DWI stratified into predefined time intervals between the onset of visual impairment and MR imaging.
Distribution of DWI and sensitivity of retinal diffusion restrictions in patients with central retinal artery occlusion according to the onset-to-MR imaging time intervals (reader 1: white columns; reader 2: gray columns). Double asterisks indicate P < .01; triple asterisks, P < .001.
DISCUSSION
Since our first description of RDR on standard stroke DWI, it remained unclear to what extent clinical features and technical MR imaging parameters contributed to the limited sensitivity (0.7–0.75) observed in our small retrospective cohort of 20 patients with CRAO,9 especially, because further scientific literature on RDR in CRAO up until now has been limited to incidental reports.10⇓-12
In this study, we retrospectively investigated 127 patients, which, as of today, constitute the largest cohort of retinal DWI in CRAO. Although we found slightly lower overall sensitivity (reader 1: 0.67, reader 2: 0.63) for RDR in CRAO than previously reported,12 sensitivity increased up to 0.79 (reader 1) in DWI performed within 24 hours after onset of vision loss.
It is conceivable that the presence of RDR in CRAO, similar to cerebral ischemia, indicates cytotoxic edema developing on disruption of cellular ion homeostasis through failure of adenosine triphosphate–dependent membrane pumps with consecutive intracellular shifts of water.17,18 We found a direct association of RDR and retinal edema because patients with fundoscopic absence of retinal edema (identified through retinal opacity and/or cherry-red spot) on admission were more likely to lack RDR on DWI.
We observed a tendency of RDR to be more frequent in patients with severe visual impairment, though differences did not reach statistical relevance. This seems conceivable because the severity of visual impairment does not necessarily depend on the extent of retinal ischemia alone but on whether the foveal region is affected or spared, eg, in a patent cilioretinal artery. A focal macular ischemia may, therefore, lead to extensive visual impairment, while the total area of retinal ischemia remains small, which, in turn, may affect the visibility of RDR in CRAO. Most interesting, the absence of RDR was more frequent in patients with complete restitution of VA on discharge, linking RDR to clinical severity in CRAO.
Our study is the first to investigate the time-dependency of RDR in CRAO, which we identified in most patients with CRAO with DWI performed within 24 hours up to 1 week after the onset of vision loss. On the contrary, our data suggest that the sensitivity of RDR declines 1 week after the onset of CRAO. Thus, diffusion restrictions in CRAO seem to occur early and do not show a delay as observed in small brain stem and cerebellar infarctions.19⇓-21 Most interesting, 3 patients had DWI performed within the hyperacute phase of CRAO (1 hour, 2 hours, and within 2–5 hours after the onset of visual impairment), 2 of whom already showed RDR. This finding indicates that by principle, RDR may be identified in patients with CRAO presenting within the suggested time window for thrombolytic therapy.22 The cross-sectional design of our analysis necessitates further research on the time-dependency of RDR, eg, within the scope of a longitudinal investigation.
A substantial proportion of our cohort of patients with CRAO had concurrent cerebral infarction on DWI (28%), irrespective of RDR. This observation is in accordance with previous studies, which found acute ischemic stroke in 19%–28% of patients and indicates the causative role of proximal embolism in nonarteritic CRAO.9⇓-11,23
This study is limited by its retrospective design and the absence of a control cohort, which increase the risk of observer bias for our diagnostic accuracy assessment of RDR. However, in our previous study, we investigated standard test quality criteria and interrater agreement for RDR using a control cohort of patients with stroke and 2 blinded readers.12 Assessment of RDR by standard DWI was possible with high specificity (0.80–1.00) and negative predictive value (0.76–0.80), with only very few cases of DWI changes falsely attributed to controls or the wrong eye of patients with CRAO. The overall sensitivity and interrater reliability for RDR in CRAO reported here are in accordance with the results from our previous study. Additionally, we did not perform ADC value measurements due to technical limitations, foremost the subvoxel dimension of the retinal thickness (between 200–400 µm), which results in considerable partial volume averaging effects, which we specified previously.12 As a consequence, the magnitude of diffusion restriction (ADC reduction) secondary to cytotoxic edema on one hand and a potential T2 shinethrough component (ADC elevation) secondary to vasogenic edema cannot be determined precisely with our methodology. However, we performed a visual qualitative evaluation of the ADC maps and were able to confirm true diffusion restriction in a substantial proportion (64.8%) of cases with positive findings on DWI by corresponding low signal.
Our data did not allow a reliable assessment of visual outcome through visual acuity and/or perimetric visual field testing because ophthalmologic follow-up examinations were not reliably documented in our data base. However, cases of complete restitution of visual acuity at the time of discharge from the hospital were specified in our medical documentation and, hence, used for analysis.
All patients underwent routine brain MR imaging not optimized for the visualization of the retina and surrounding structures. Furthermore, DWI was acquired using different scanners, receive coils, and sequences. We acknowledge that the heterogeneous technical realization of image acquisition is a limitation of our study. Yet, it documents that RDR in CRAO are visible in a variety of routine MR imaging setups. Future investigations should focus on the technical improvement of MR imaging protocols in CRAO. In this regard, more recent developments in DWI sequence techniques such as readout-segmented DWI, small-FOV DWI, and fast spin-echo radial acquisition DWI sequences have already been proved valuable for imaging of various orbital and skull base pathologies, including intraocular masses, optic neuritis, and cholesteatoma.24⇓-26 By achieving either higher signal-to-noise ratios or reduced distortion, movement, and susceptibility artifacts, these techniques may improve the diagnostic potential of MR imaging for acute CRAO compared with conventional EPI sequences by better exploiting and visualizing the rather small amount of signal provided by the delicate retinal cell layers.
A number of case reports have described the occurrence of RDR in other retinal pathologies, such as retinal necrosis in herpes simplex virus type 1 infection,13 subretinal abscess,27 and ophthalmic vein thrombosis due to cavernous sinus thrombophlebitis.28 Hence, further studies are warranted to investigate the specificity of RDR to identify CRAO in patients presenting with sudden, painless, monocular visual impairment.
CONCLUSIONS
Our retrospective analysis of 127 patients confirms RDR as a regular finding in acute CRAO and, for the first time, reveals the time course of RDR with good detectability in DWI performed within 24 hours up to 1 week after onset of visual impairment. Visibility of RDR on DWI may indicate irreversible retinal damage due to cytotoxic edema and is dependent on the clinical course of the disease.
Footnotes
L.A. Danyel is participant in the BIH-Charité Clinician Scientist Program funded by the Charité–Universitätsmedizin Berlin and the Berlin Institute of Health.
References
- Received October 27, 2020.
- Accepted after revision April 4, 2021.
- © 2021 by American Journal of Neuroradiology