Abstract
BACKGROUND AND PURPOSE: New imaging techniques such as hybrid imaging of ultrasound and FDG-PET/CT are available but not yet investigated for node staging. The aim of the study was to evaluate the feasibility and added diagnostic value of real-time image-fused ultrasound-guided fine-needle aspiration with FDG-PET/CT data for node staging.
MATERIALS AND METHODS: Ninety-six patients who were referred for cervical lymph node staging with FDG-PET/CT before ultrasound were prospectively included. After routine ultrasound-guided fine-needle aspiration, all FDG-PET-positive nodes were marked on FDG-PET/CT, and real-time image fusing of ultrasound and FDG-PET/CT was performed using the electromagnetic navigation system PercuNav. Already-punctured nodes were confirmed to be PET-positive, and additional fused-ultrasound-guided fine-needle aspiration was performed in previously missed PET-positive nodes.
RESULTS: Of 96 patients, 87 (91%) patients had suspicious nodes requiring fine-needle aspiration cytology. Ultrasound-guided fine-needle aspiration was performed in 175 nodes. Cytology was inconclusive in 9/175 (5%) nodes, and 85/166 (51%) nodes were malignant. Target planning was performed in 201 PET-positive nodes; 195/201 (97%) of those nodes were fused successfully. Twenty of 175 ultrasound-guided fine-needle aspiration nodes turned out to be FDG-PET-negative, and 149/175 (85%) of the fused ultrasound-guided fine-needle aspiration nodes were confirmed to be FDG-PET-positive. Of 201 PET-positive nodes, 46 (23%) were additionally identified, and fused ultrasound-guided fine-needle aspiration was performed. Cytology was inconclusive in 4/46 nodes (9%), and 13/42 (31%) nodes were malignant.
CONCLUSIONS: Real-time ultrasound image fusion with FDG-PET-positive nodes is feasible in cervical lymph nodes, and fused ultrasound-guided fine-needle aspiration increases the number of malignant nodes detected.
ABBREVIATIONS:
- cN0
- clinically node-negative neck
- FNAC
- fine-needle aspiration cytology
- HNC
- head and neck cancer
- ND
- neck dissection
- TNM
- Tumor, Node, Metastasis
- US
- ultrasound
- USgFNAC
- ultrasound-guided fine-needle aspiration cytology
The Tumor, Node, Metastasis (TNM) stage in head and neck cancer (HNC) is important for prediction of prognosis and stratification of treatment. Besides physical examination, imaging plays a crucial role in defining the TNM stage, assessing tumor volume and nodal involvement.1,2 Nodal staging with CT and MR imaging is limited with a per-patient sensitivity ranging from 73% to 87% for CT and 70% to 74% for MR imaging.3 In the clinically node-negative neck (cN0), the sensitivity ranges from 14% to 80% for CT and from 29% to 85% for MR imaging; on average, the sensitivity is in the range of 40%–60%.4 Molecular imaging of glucose metabolism with FDG-PET/CT has a higher per-neck-level sensitivity for detection of regional nodal metastases in patients with primary head and neck squamous cell carcinoma, with a sensitivity of up to 84% and a specificity up to 96%.5,6 However, for cN0, an overall sensitivity of 21.4% and specificity of 98.4% have been reported.7 In comparison with sentinel node biopsy in cN0 head and neck cancer, MR imaging and CT are not effective in predicting whether prophylactic neck dissection (ND) can be safely avoided, and the sensitivity of FDG-PET/CT may still not be adequate.8 In clinical practice, ultrasound-guided fine-needle aspiration cytology (USgFNAC) plays an important role, not only as an upfront imaging technique for the neck but also to determine the diagnosis in equivocal lymph nodes on CT, MR imaging, or FDG-PET/CT.9 The sensitivity of USgFNAC in patients with clinically suspicious nodes has been reported to be 88%,10 but the sensitivity drops significantly to 39% in patients with a cN0.11 Apart from minimizing the chance of sampling errors, selection of the most suspicious nodes that need aspiration is a major challenge.12 Selection of nodes by FDG-PET/CT standard uptake value might improve selection of the most suspicious nodes for fine-needle aspiration cytology (FNAC).
Due to technical improvements, it is possible to fuse real-time ultrasound (US) with cross-sectional imaging techniques such as with PET/CT, CT, or MR imaging.13 Fusion of US with FDG-PET/CT to guide FNAC of nodes can potentially improve the identification and detection of malignant nodes. The aim of our study was first to evaluate the feasibility of US real-time fusion with FDG-PET/CT data for fused image guidance of fine-needle aspiration in suspicious neck nodes and second to evaluate whether it leads to a more accurate detection of malignant nodes.
MATERIALS AND METHODS
Patient Population
We prospectively included 96 patients (Table 1) who were referred for USgFNAC with prior FDG-PET/CT and met one of the following criteria: histopathologically proved HNC and histologically proved lymph node metastasis with an unknown primary or suspicious head and neck lesion, not yet proved to be malignant. All data were analyzed retrospectively. After routine ultrasound and USgFNAC, real-time fusion of ultrasound and FDG-PET/CT took place to confirm USgFNAC nodes to be PET-positive and to perform additional fused-USgFNAC of missed FDG-PET-positive nodes that would change the N stage. This study was approved by the Netherlands Cancer Institute institutional medical ethics committee (METC16.0745) and the Netherlands Cancer Institute institutional review board (IRBd20-126). All retrospective medical data/biospecimen studies at the Netherlands Cancer Institute have been executed pursuant to Dutch legislation and international standards. Before May 25, 2018, national legislation on data protection applied, as well as the International Guideline on Good Clinical Practice. From May 25, 2019, we also adhered to the General Data Protection Regulation. Within this framework, patients are informed and have always had the opportunity to object or actively consent to the (continued) use of their personal data and biospecimens in research. Hence, the procedures comply with both national and international legislative and ethical standards.
Diagnosis of all patientsa
Table 1 shows the diagnosis in number and percentages of all included patients and the number and percentages of patients with head and neck squamous cell carcinoma. Table 2 shows an overview of the treatment.
Combination of treatments of all 96 patients
FDG-PET/CT Imaging
For FDG-PET/CT, images were acquired using a Gemini TF scanner (Philips Healthcare). Patients fasted for 6 hours and were hydrated before administration of FDG. Diabetes mellitus needed to be regulated adequately. The plasma glucose level was required to be below 10 mmol/L. A dose of 190–240 MBq was administered depending on body mass index. FDG-PET images of the head and neck area were acquired for 3 bed positions of 3 minutes each. They were reconstructed to 2-mm isotropic voxels. Low-dose CT was acquired for attenuation correction and anatomic orientation with 40 mAs and 2-mm slices. In addition, images of the neck were acquired. All FDG-PET/CT images were interpreted by an experienced nuclear physician for clinical staging, and this report was available for interpretation of involved nodes in this study.
Ultrasound and FNAC
The FDG-PET/CT data were imported into the US device (EPIQ 7 G; Philips Healthcare) before the routine procedures. First, routine US evaluation and routine USgFNAC with a 21-ga needle without use of FDG-PET/CT data were performed. All USgFNACs were performed by 1 radiologist (P.K.d.K.-D.) who has 10 years of USgFNAC experience in HNC. She was aware of the clinical information and available imaging data, including FDG-PET/CT, before performing US. FNAC was performed in 1 or 2 neck levels ipsilateral and sometimes contralateral in suspicious nodes in the levels at most risk, corresponding to the site of the primary tumor, as well as in suspicious nodes at the lowest neck level of each side. Nodes were aspirated at a short-axis diameter of >1 cm or <1 cm and showed loss of a fatty hilum or showed a thickened or asymmetric cortex or round shape. Also, nodes described in the MR imaging or FDG-PET/CT to be borderline or suspicious were aspirated when identified.
Immediately after the routine US and USgFNAC procedures, real-time image fusion of US and FDG-PET/CT using the electromagnetic navigation system PercuNav (Philips Healthcare; FDA- and Conformité Européenne–approved and available worldwide), installed on the same US diagnostic system, took place by the same reader (P.K.d.K.-D.). US was performed using either a L12-5 or an eL18-4 probe with an integrated electromagnetic tracker (both from Philips Healthcare). During the image-fusion steps, a bracket and the respective electromagnetic tracker were added to the L12-5 probe so it could be used with the PercuNav system. The PercuNav setup was used according to the manufacturer’s manual. The patient reference tracker was placed on the forehead of the patient and held in place with tape. The field generator was positioned above the patient’s neck using a metallic arm. The initial fusion between real-time US and FDG-PET/CT was performed by identifying the thyroid on both modalities and using the “matched plane” function on the system (Fig 1).
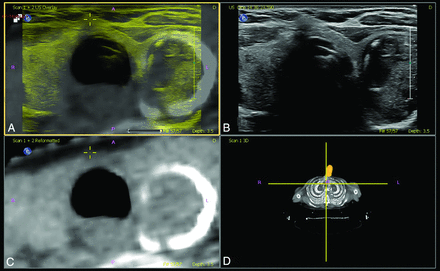
Matched plane fusion, manual correction. A, Overlay US (yellow) and CT (gray). B, US image. C, Reformatted CT image. D, Volume representation of CT image and probe location.
Additional manual corrections to the fusion were made by identification of known anatomic structures such as the hyoid bone, submandibular gland, and carotid artery bifurcation. Then a target was created for each FDG-PET/CT–positive lymph node using the target planning function (including nodes with a low risk of malignancy and low standard uptake values) (Fig 2). At this time, the radiologist identified the PET-positive nodes targeted on FDG-PET/CT and verified whether USgFNAC had already been performed (Fig 2). In patients with multiple FDG-PET–positive nodes, a selection was made on the basis of level, size, and standard uptake value for fused aspiration. In case the node had not been previously aspirated, fused-USgFNAC was performed.
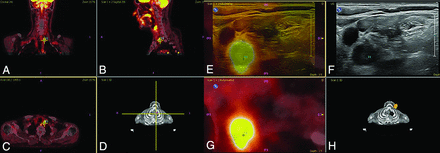
Target planning and real-time fusion of FDG-PET-positive lymph nodes to identify PET-positive nodes. A, Coronal view. B, Sagittal view. C, Axial view. D, Volume representation of FDG-PET/CT image and probe location, PET-positive nodes where targeted, and real-time image fused with ultrasound. E, Overlay US (yellow) and PET/CT (gray). F, US image. G, Reformatted PET/CT image. H, Volume representation of the CT image and probe location. Fusion of PET and CT and target planning took place using the electromagnetic navigation system PercuNav. First, routine ultrasound and routine USgFNAC were performed. Second, ultrasound and FDG-PET-positive nodes were real-time fused. USgFNAC in PET-positive nodes was confirmed, and additional fused-USgFNAC of missed PET-positive nodes was performed.
Reference Standard
The surrogate reference standard was the pathologic result of FNAC. Because only 19/96 patients underwent ND and 1/96 patients underwent sentinel node biopsy, this series was too small to reliably estimate the sensitivity and specificity of USgFNAC, fused-USgFNAC, or FDG-PET/CT. If available, histopathology of the ND specimen was used as a reference standard and the pN stage was compared with the pN stage of USgFNAC and fused-USgFNAC (Online Supplemental Data).
Statistical Analysis
A 2-sample t test was used to compare the mean size of nodes between USgFNAC and fused-USgFNAC. By means of the Mantel-Haenszel test, the detection rate of malignant nodes in USgFNAC and fused-USGFNAC was compared. The χ2 test was used to compare the accuracy of the N stage found with USgFNAC and fused-USgFNAC in relation to the cytology results.
RESULTS
Nine of 96 patients (9%) who were selected for the study did not have any FDG-PET–positive nodes or suspicious nodes on US. In the remaining 87/96 (91%) patients, a total of 221 lymph nodes were aspirated. The median number of aspirated nodes per patient was 2 (range, 1–5).
USgFNAC
One hundred seventy-five of 221 lymph nodes were aspirated during routine US; the smallest nodes were 4 mm, and the mean minimal axial diameter was 11.7 mm. At USgFNAC, 9 of 175 nodes (5%) were inconclusive at cytology, and 85 of 166 (51%) nodes were malignant.
Fused-USgFNAC
Target planning was performed in 201 PET-positive nodes. Fusion was technically successful in 195/201 (97%) FDG-PET-positive nodes.
One hundred forty-nine of 175 (85%) USgFNAC nodes were confirmed to be FDG-PET-positive. Cytology was inconclusive in 9/175 (5%). Of the remaining 140 confirmed PET-positive nodes, 83 (59%) nodes proved to be malignant. At fusion, 20 of the USgFNAC nodes proved to be FDG-PET-negative, and only 1 of those nodes was malignant.
On the basis of fusion, 46/201 (23%) FDG-PET-positive nodes were additionally identified and fused-USgFNAC was performed; the smallest nodes were 3 mm, and the mean minimal axial diameter was 6.3 mm (range, 3–16 mm), which was significantly smaller than in routine USgFNAC (P value < .001) (Table 3).
Size and location of additional fused-USgFNAC nodes
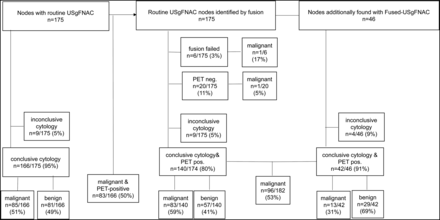
At cytology, 4/46 nodes (9%) were inconclusive and 13 of 42 (31%) nodes proved to be malignant. Added fused-USgFNAC increased the number of proved malignant nodes from 85 to 98 (15%). Due to additional fused-USgFNAC, the percentage of proved PET-positive malignant nodes changed from 83/166 (50%) to 96/182 (53%) (statistically insignificant P value < .29; flow chart, Fig 3).
Flow chart results of routine USgFNAC and fused-USgFNAC. Pos. Indicates positive.
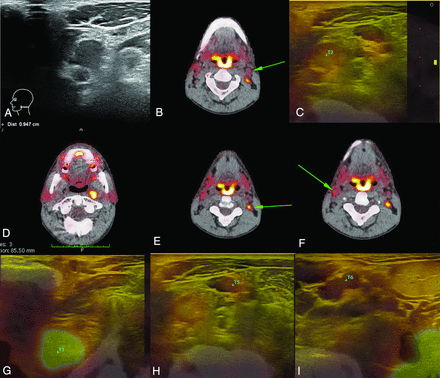
With fused-USgFNAC, the pN-stage was upgraded in 8/87 (9%); in 2/8, from node-negative neck stage to N1 stage; and in 1/8, from N1 to N3 stage (Fig 4).
Change of N stage after additional fused-USgFNAC. The patient presented with cT3N0 oropharyngeal squamous cell carcinoma. A, Results of routine USgFNAC N1. B and C, PET/CT of the same node, controlled by image fusion. D–F, Additional nodes on PET/CT; all nodes have been fused, and fused-USgFNAC was performed. G, The deep parapharyngeal node was missed at routine ultrasound and only recognized after fusion. H, A PET-positive node with a normal appearance on routine ultrasound. I, Fused-USgFNAC-proved benign PET-positive contralateral node. Cytologically proved pN stage after fused-USgFNAC was pN2b, while it was N1 with USgFNAC and N2c on PET/CT. The green arrows point to the PET-positive nodes.
N staging with USgFNAC and fused-USgFNAC was not significantly different (P value < 0.1).
ND was performed in 19/96 (20%) patients, and sentinel node biopsy, in 1/96 (1%). A total of 610 nodes were removed, and in 11 of these 20 patients, 52 metastases were present. With USgFNAC, the pathologic result was pN0 stage in 2 of these patients, while it was pN2b stage and pN2c stage in the ND. With fused-USgFNAC, it was pN1 in both of those patients.
DISCUSSION
Our study shows that real-time US image fusion with FDG-PET/CT is feasible and allows accurate US identification of the FDG-PET-positive nodes. In our routine FNAC procedure, clinical information and imaging data including FDG-PET/CT are also available but not fused. In this study, we sought to demonstrate the additional effect of using fused USgFNAC, and this was demonstrated by an increase of pathologically confirmed malignant nodes from 85 without fusion to 98 with additional fusion. Accurate nodal staging is a major determinant for treatment decisions in head and neck squamous cell carcinoma.14
When we comparing fused-USgFNAC pN-stage with USgFNAC, 8/87 (9%) patients with pN stage were upgraded though N staging with USgFNAC and fused-USgFNAC was not significantly different.
Currently, the neck is staged by clinical palpation, CT, MR imaging, FDG-PET/CT, and/or USgFNAC in the case of suspicious nodes. For most imaging modalities, apart from irregularities and shape, the minimal axial diameter is one of the most important criteria for suspicious nodes to be selected for aspiration.15 For FDG-PET/CT, the standard uptake value is a criterion for metastasis. All these imaging techniques fail to accurately detect very small metastases, leading to a sensitivity in the clinically node-negative neck on the order of 40%–60%8 due to the higher frequency of small-sized nodes. Furthermore, FDG-PET/CT is limited in resolution and glucose uptake in small nodal metastases.16 The main reason for this feature is that 25% of the metastases in clinically node-negative necks are <3 mm and, thus, will not be easily detected by any individual imaging technique.11
Treatment decision-making in HNC relies on imaging, emphasizing the need for high sensitivity to depict nodal disease.17,18 Not only the extent of neck dissection but also the fields and doses of radiation therapy are guided by imaging results. As with FDG-PET/CT, subtle metabolically active lymph nodes are very difficult to characterize, and a subsequent USgFNAC is performed to make the final verdict. USgFNAC of the wrong lymph node or wrong part of the lymph node will lead to false-negative USgFNAC results, with undertreatment as a result.18 We were able to show that real-time US image fusion with FDG-PET/CT is feasible. Within the 201 FDG-PET-positive nodes, fusion failed in only 6 nodes (8%), and this was mainly at the starting period when we were still in the midst of our learning curve. Real-time US image fusion with FDG-PET/CT is an excellent method to increase the reliability of the FDG-PET/CT results. Especially in borderline, small FDG-PET/CT-positive lymph nodes, fusion with US can increase the yield of fused-USgFNAC and diminish sampling errors. Particularly for small nodes, real-time US image fusion with FDG-PET/CT can improve the sensitivity of ultrasound and the specificity of FDG-PET/CT and lead to a higher detection rate of malignant nodes. Although in both fused-USgFNAC and USgFNAC, the smallest FDG-PET-positive malignant lymph nodes were 4 mm, the mean minimal axial diameter of the tumor-positive nodes of fused-USgFNAC (7.8 mm) was significantly smaller than that of USgFNAC (13.4 mm).
Although additional fused-USgFNAC increased the number of confirmed malignant nodes from 85 to 98, the detection rate of malignant PET-positive nodes increased only from 51% to 53%, which was not significant. This can largely be explained by the smaller size of the additional fused-USgFNAC nodes, indicating an increase in sensitivity in small nodes. The N stage was upgraded in 8/87 (9%) patients.
Because we do not have definitive pathology of all aspirated lymph nodes, we cannot determine whether the FDG-PET/CT was false-positive or the aspiration was false-negative for the cases with negative aspirates. In addition, in 3/21(14%) patients with insufficient FNAC results, malignant nodes in elective neck dissection were present, which suggests that every FNAC with an insufficient result should be repeated. On FDG-PET/CT, smaller nodes are more often borderline FDG-positive nodes, which can lead to a problem in diagnosis. A visible slightly higher metabolism can be caused by a metastasis as well as by inflammation. Consequently, the specificity of a maximum standard uptake value between 2 and 3 at FDG-PET/CT is quite low and can be increased by adding fused-USgFNAC. On the other hand, the specificity of USgFNAC is almost 100%, so a combination of using FDG-PET/CT with a lower threshold and fused-USgFNAC might improve the sensitivity of the USgFNAC.
The selection of nodes for aspiration in HNC is a difficult issue. Size and location are the most important selection criteria. This study was meant to see whether image fusion with FDG-PET/CT is a helpful tool for node selection to provide FNAC. Especially in small lymph nodes with limited uptake, this technique could be added to USgFNAC. Only 1 patient with negative FDG-PET/CT findings had a suspicious node on ultrasound, which proved to be malignant. In all other patients, after fusion, all the nodes that underwent routine USgFNAC were, to some extent, FDG-PET-positive, so the current criteria for aspiration largely overlap with the glucose uptake at FDG-PET/CT. One could argue whether aspirating from more and smaller nodes without PET guidance would increase the sensitivity irrespective of adding FDG-PET/CT, but selection criteria only guided by size and shape are not very accurate, and borderline glucose uptake may well be more reliable.
Although in the prostate and liver, real-time fused image-guided biopsies are already used clinically,19,20 the technique has its limitations in head and neck imaging. The mobility of the neck makes fusion much more difficult. Autofusion is not successful. Manual fusion and fusion corrections on the different levels of the neck must been done. To get a reliable accurate fusion, the radiologist must be well-trained. Because fused image-guided FNAC is time-consuming, with an additional 10–15 minutes of examination time, it should be used as a problem-solving tool in small, borderline FDG-PET-positive nodes, which are difficult to identify on routine USgFNAC. As far as we know, this is the first larger study of fused USgFNAC in HNC; therefore, the reproducibility is not known; and because there was only 1 observer, the interobserver variability is also not known. No reliable estimation of the sensitivity and specificity could be made due to the small number of patients who underwent neck dissection and sentinel node biopsy.
CONCLUSIONS
Real-time US image fusion with FDG-PET/CT and fused-USgFNAC is feasible in head and neck cancer. It can improve the detection and image-guided aspiration of suspicious nodes as visualized on FDG-PET/CT and might increase the sensitivity of USgFNAC by selecting smaller FDG-PET-positive borderline nodes for fused-USgFNAC. Because fused-USgFNAC is time-consuming, it should be used as a problem-solving tool in small, borderline FDG-PET-positive nodes, which are difficult to identify on routine USgFNAC.
Acknowledgments
The authors would like to acknowledge Pedro Sanches, PhD, and Frans Gleuwink (Philips Benelux, Boschdijk 525, 5621JG Eindhoven) for providing knowledge and training on PercuNav image fusion (MR imaging, CT, PET).
Footnotes
Disclosures: Petra K. de Koekkoek-Doll—UNRELATED: Employment: radiologist Netherlands Cancer Institute. Jonas Castelijns—RELATED: Travel/Accommodations/Meeting: Employment: radiologist Netherlands Cancer Institute.
References
- Received June 24, 2020.
- Accepted after revision September 29, 2020.
- © 2020 by American Journal of Neuroradiology