Abstract
BACKGROUND AND PURPOSE: Mechanical thrombectomy in acute ischemic stroke within 6 hours of symptom onset is effective and safe. However, in many patients, information on the beginning of symptoms is not available. Patients can be divided into those with wake-up stroke and daytime-unwitnessed stroke. Evidence on outcome and complications after mechanical thrombectomy in wake-up stroke and daytime-unwitnessed stroke is rare. A potential beneficial effect of mechanical thrombectomy in selected patients with wake-up stroke or daytime-unwitnessed stroke is suspected.
MATERIALS AND METHODS: We analyzed 1073 patients with anterior circulation stroke undergoing mechanical thrombectomy between 2010 and 2016. Patients with wake-up stroke and daytime-unwitnessed stroke were compared with controls receiving mechanical thrombectomy as the standard of care. We assessed good functional outcome (mRS ≤ 2 at 3 months), mortality rates, and frequencies of symptomatic intracranial hemorrhage. Subgroup analyses tried to detect influences of patient selection via further imaging modalities (MR imaging, CTP; wake-up stroke [advanced], daytime-unwitnessed stroke [advanced]) on outcome and safety.
RESULTS: There was no significant difference in good functional outcome between patients with wake-up stroke and controls (35.9% versus 38.3%, P = .625). Outcome in patients with daytime-unwitnessed stroke was inferior compared with controls (27.3%, P = .007). Groups did not differ in all-cause mortality at day 90 (P = .224) and the rate of symptomatic intracranial hemorrhage (P = .292). Advanced imaging improved the frequency of good functional outcome (non-wake-up stroke [advanced] versus wake-up stroke [advanced]: OR, 2.92; 95% CI, 1.32–6.45; non-daytime-unwitnessed stroke [advanced] versus daytime-unwitnessed stroke [advanced]: OR, 2.09; 95% CI, 1.03–4.25) with an additional reduction in all-cause mortality (non-daytime-unwitnessed stroke [advanced] versus daytime-unwitnessed stroke [advanced]: OR, 0.42; 95% CI, 0.20–0.88).
CONCLUSIONS: Mechanical thrombectomy in selected patients with wake-up stroke allows a good functional outcome comparable with that of controls. Outcome after mechanical thrombectomy in daytime-unwitnessed stroke seems to be inferior compared with that in controls. Advanced imaging modalities may increase the frequency of good functional outcome in both patients with wake-up stroke and daytime-unwitnessed stroke.
ABBREVIATIONS:
- aTE
- aspiration thrombectomy
- DUS
- daytime-unwitnessed stroke
- mTE
- mechanical thrombectomy
- WUS
- wake-up stroke
Mechanical thrombectomy (mTE) in acute ischemic stroke due to embolic large-vessel occlusion has been shown to be effective and safe. Several randomized controlled trials have demonstrated the superiority of mTE in combination with intravenous thrombolysis compared with intravenous thrombolysis alone.1⇓⇓⇓–5 Subsequently, specific recommendations for patient selection and execution of mTE and/or aspiration thrombectomy (aTE) were implemented (eg, initiation within 6 hours after symptom onset).6 However, in many patients, information on the beginning of stroke symptoms is not available. They might therefore be excluded from beneficial endovascular therapy. Evidence on mTE in a prolonged time window is inconsistent, and data on the efficacy and safety of mTE in patients with unknown symptom onset are rare.7⇓–9
Materials and Methods
From our ongoing prospective single-center stroke registry, consecutive patients treated with mTE/aTE between January 2010 and December 2016 were considered for this retrospective noninterventional analysis. Patients were either primarily treated in our center (Neurozentrum, Klinikum Stuttgart) or secondarily transferred from hospitals in surrounding cities.10 Nonetheless, enrollment was based on the initial intention to treat patients via mTE/aTE. There was no secondary triage, selection procedure, or additional imaging before the intervention. The study was approved by the local institutional review board.
Study Population
Patients with an anterior circulation stroke due to an occlusion of the ICA, the intracranial carotid bifurcation, or an M1 or M2 branch of the MCA were included. Patients with an occlusion of an MCA M3 branch, the anterior cerebral artery, or the posterior circulation were excluded. In case of an initial proximal vessel occlusion that was later found recanalized during angiography (spontaneously or after intravenous thrombolysis), datasets were removed from further analysis. We did exclude patients who were not treated according to current recommendations (eg, because of a delayed treatment onset) unless they were classified as having wake-up stroke (WUS) or daytime-unwitnessed stroke (DUS).6 Further exclusion criteria were the following: stent angioplasty without mTE/aTE due to high-grade intra- or extracranial stenosis or dissection because of anticipated differences in clinical outcome; the application of older generation stent retrievers and aspiration systems not used in recent randomized controlled trials (the following stent retrievers and aspiration systems were included: Solitaire FR, Medtronic, Minneapolis, Minnesota; pREset, phenox, Bochum, Germany; ACE aspiration catheters, Penumbra, Alameda, California; and Sofia, MicroVention, Tustin, California); and the lack of a 3-month follow-up. Datasets with inconsistent information that could not be verified were excluded.
Patients treated within 6 hours of symptom onset (according to current guidelines) were included in the control group (C). Because of expected differences in pathophysiology and outcome, patients with an unknown symptom onset were divided in the following manner: 1) WUS, occurring out of sleep in the early morning hours; 2) DUS.11⇓–13 WUS was defined as stroke symptoms being present during awakening. Patients had to be asymptomatic when going to sleep and during the night. DUS included patients who were asymptomatic while waking up or had recognized symptoms at some point during day or night. Patients without information on “last seen well” who could not be specified as having WUS by the assessing neurologist were subsumed in DUS.
Subgroup analyses were conducted using consecutive steps in patient selection: 1) further imaging modalities with CTP or MR imaging allowing patient selection due to a mismatch concept (CBV versus TTP or MTT in CTP, FLAIR-DWI mismatch in MR imaging; WUS[advanced], DUS[advanced]); and 2) advanced imaging with MR imaging only (WUS[mri], DUS[mri]).14⇓⇓⇓⇓–19 Examples of mismatch in both MR imaging and CTP are shown in On-line Figs 1–3. The decision to perform advanced imaging was made by the respective stroke specialist. In patients without CTP or MR imaging, we routinely opted for mTE when there was a proved vessel occlusion (eg, in CTA, hyperdense vessel sign), no major demarcation of infarcted tissue on plain CT, or an ASPECTS score of ≥4. There was no fixed CTP protocol (eg, quantitative measurement or thresholds defining mismatch). Information on a possible mismatch was reported by the referring hospital or our neuroradiology department. The results were validated by the neurointerventionalist before endovascular therapy. WUS[mri] and DUS[mri] were subsequently compared with a selected control group (C[mri]) with MR imaging as the initial imaging technique. To detect a possible effect of imaging selection on outcome, we compared patients with WUS and DUS in subgroups 1 and 2 with the remaining patients with WUS or DUS not part of the respective group (eg, WUS[mri] versus non-WUS[mri]).
Outcome Measures
Primary outcome measure was mRS at day 90, with mRS 0–2 indicating good functional outcome. Secondary outcome measures were the following: 1) development of a symptomatic intracranial hemorrhage according to the Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) criteria (parenchymal hemorrhage type 1 or 2, SAH, or intraventricular hemorrhage within 24 hours after mTE with a deterioration in the NIHSS score of ≥4 points or leading to death)20; 2) in-hospital mortality; and 3) all-cause mortality at day 90.
Data Collection
Information on age, sex, medical history, stroke onset, NIHSS, imaging technique, mRS score, and stroke etiology was drawn from referral letters, admission notes, or discharge papers. Imaging times were stored in our PACS. Periprocedural information (eg, TICI scores) was documented by the neuroradiology department. Follow-up data were collected by our study nurse via telephone calls.
Statistical Analysis
Numeric baseline characteristics were described in medians (quartiles) or means (SDs). Categoric baseline parameters were described in frequencies. For comparing groups, the Fisher exact test or the χ2 test was used for categoric parameters. Numeric parameters were analyzed with the Kruskal-Wallis-test or the Mann-Whitney U test as appropriate. Dichotomized outcome (head-to-head comparison of groups) was evaluated in a univariate logistic regression model adjusting for possible confounders (based on literature research; baseline-NIHSS, age, ICA occlusion, stroke etiology, imaging-to-groin time, diabetes mellitus, and hypertension). A P value < .05 was considered statistically significant. STATA/IC 13.1 for Windows (StataCorp, College Station, Texas) was used for statistical analysis.
Results
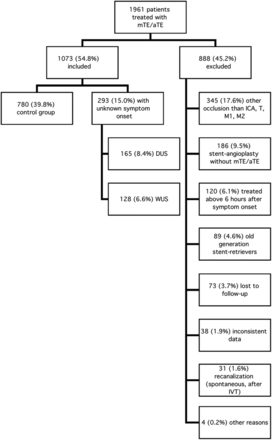
Between January 2010 and December 2016, one thousand nine hundred sixty-one patients were treated with mTE/aTE, 888 patients (45.2%) did not meet the predefined inclusion criteria (Fig 1), and 1073 patients (54.8%) would eventually be analyzed. Most (n = 780, 72.7%) formed the control group. In 293 patients (27.3%), symptom onset was unclear. One hundred twenty-eight patients (11.9%) were categorized as having WUS; and 165 (15.4%), DUS.
Flowchart depicting patient selection according to predefined inclusion and exclusion criteria. IVT indicates intravenous thrombolysis.
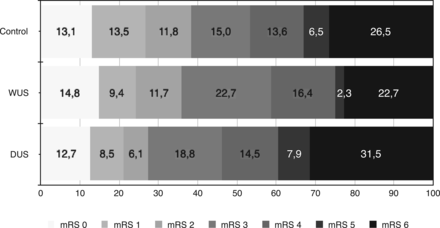
The baseline characteristics are shown in On-line Table 1. There was no difference in the frequency of good functional outcome (mRS ≤ 2) between patients with WUS and controls (35.9% versus 38.3%, P = .625; Table 1). Good functional outcome was reduced in those with DUS compared with controls (27.3%, P = .007). WUS and DUS did not differ significantly (P = .127). Figure 2 illustrates the distribution of mRS scores at day 90. In-hospital mortality (C: 18.9%; WUS: 12.6%; DUS: 23.3%; P = .067) and all-cause mortality at day 90 (C: 26.5%; WUS: 22.7%; DUS: 31.5%; P = .224) did not differ significantly. The rate of symptomatic intracranial hemorrhage was similar in all groups (P = .292, Table 1). Results did not change in the head-to-head comparison of groups adjusted for the abovementioned confounders (good functional outcome; WUS versus C: OR, 0.74; 95% CI, 0.48–1.16; P = .193; DUS versus C: OR, 0.49; 95% CI, 0.31–0.79; P = .003; data not shown).
Primary and secondary outcome parameters
Distribution of mRS scores at 90 days.
Sixty-eight of the 128 patients with WUS (53.1%) underwent CTP or MR imaging (DUS: n = 63; 38.2%). MR imaging was performed in 36.7% of patients with WUS (n = 47) and in 31.5% of those with DUS (n = 52). In WUS[advanced], mismatch was present in 52 patients (76.5%; DUS[advanced]: n = 46, 73.0%; WUS[mri]: n = 36, 76.6%; DUS[mri]: n = 37, 71.2%). Subgroup analyses based on imaging selection are summarized in Table 2. When we compared WUS[advanced] and DUS[advanced] with unselected controls, there was no statistically significant difference in good functional outcome (C: 38.3%; WUS[advanced]: 47.1%; DUS[advanced]: 36.5%; P = .344). In-house mortality was reduced significantly in WUS[advanced] compared with controls (7.5% versus 18.9%, P = .019). Similar results were seen in patients with WUS[mri] and DUS[mri] (Table 2).
Subgroup analysis—patient selection via imaging modalities
When we compared controls with patients in the WUS and DUS subgroups presenting with a verified mismatch only, the results did not change. Mortality rates remained stable with a nonsignificant increase in the percentage of good functional outcome (WUS[advanced]: 51.9%; DUS[advanced]: 39.1%; WUS[mri]: 52.8%; DUS[mri]: 43.2%; On-line Table 2). There was no significant difference in good functional outcome among WUS[mri] (51.1%), DUS[mri] (38.5%), and C[mri] (48.8%; P = .357; Table 2). The same was true for all-cause mortality at 3 months (C[mri]: 20.0%; WUS[mri]: 12.8%; DUS[mri]: 23.1%; P = .385) and the rate of symptomatic intracranial hemorrhage (P = .875).
We did compare WUS[advanced] and WUS[mri] with WUS not part of the respective groups (non-WUS[advanced], non-WUS[mri]; Table 3). Non-WUS[advanced] versus WUS[advanced] showed an increase in the rate of good functional outcome (23.3% versus 47.1%; OR, 2.92; 95% CI, 1.32–6.45; P = .006). In non-WUS[mri] versus WUS[mri], good functional outcome increased similarly (27.2% versus 51.1%; OR, 2.80; 95% CI, 1.28–6.10; P = .008) with an additional decrease in in-hospital mortality (17.3% versus 4.3%; OR, 0.22; 95% CI, 0.05–1.04; P = .049) and all-cause mortality at day 90 (28.4% versus 12.8%; OR, 0.37; 95% CI, 0.14–1.01; P = .05). For DUS, advanced imaging provided a significant improvement in the frequency of good functional outcome (non-DUS[advanced] versus DUS[advanced]: 21.6% versus 36.5%; OR, 2.09; 95% CI, 1.03–4.24; P = .048; non-DUS[mri] versus DUS[mri]: 22.1% versus 38.5%; OR, 2.20; 95% CI, 1.06–4.55; P = .038). A significant reduction of in-hospital mortality (30.0% versus 12.7%; OR, 0.34; 95% CI, 0.14–0.82; P = .013) and all-cause mortality at day 90 (38.2% versus 20.6%; OR, 0.42; 95% CI, 0.20–0.88; P = .025) was seen in non-DUS[advanced] versus DUS[advanced].
Effect of advanced imaging on outcome and safety
Discussion
Stroke with an unknown symptom onset is frequent and accounts for up to 30% of ischemic stroke cases.11⇓–13 WUS (in which symptoms are realized during awakening) and DUS (unrealized symptom onset during daytime) can be distinguished. Endovascular therapy is currently not approved in those patients.6 On the basis of a considerable real-world dataset, we tried to detect differences in outcome among patients with WUS, DUS, and controls (excluding patients with known symptom onset presenting >6 hours after notification of symptoms). In our cohort, outcome in WUS did not differ from that in controls (mRS 0–2: 35.9% versus 38.3%), suggesting a beneficial effect of mTE in WUS. No increase in mortality rates or the frequency of symptomatic intracranial hemorrhage was observed. Functional outcome in DUS overall was significantly inferior compared with that of controls (mRS 0–2: 27.3%).
The first evidence indicating a possible beneficial effect of intravenous thrombolysis in carefully selected patients presenting in a prolonged time-window was published several years ago.21,22 Similar results were obtained for endovascular therapy in late-presenting stroke cases.23,24 From a present-day perspective, the use of older generation thrombectomy devices and small sample sizes limited generalizability. Subsequent retrospective and observational data suggested a potential beneficial effect of mTE in WUS with unknown symptom onset.7,9 However, DUS was not addressed. Recently, preliminary results of the diffusion-weighted imaging or CT perfusion assessment with Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention with Trevo (DAWN) and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) trials were presented.25⇓⇓–28 DAWN included WUS and patients “last seen well” up to 24 hours prior to endovascular therapy; DEFUSE 3 recruited patients treated between 6 and 16 hours after notification of symptoms. Both randomized controlled trials were terminated after interim analysis demonstrated superiority of the interventional group. It was proved that a strict imaging selection can identify patients eligible for mTE well after the currently approved time window. So far there is still little information on possible differences between WUS and DUS.
In our data, patient selection due to administration of CTP or MR imaging likewise led to a significant increase in the frequency of good functional outcome in patients with WUS and DUS. Consecutive steps in patient selection (1, all patients; 2, patients with CTP or MR imaging; 3, patients with MR imaging; 4, patients with documented mismatch in CTP or MR imaging; 5, patients with documented mismatch in MR imaging) increased the frequency of good functional outcome in both WUS and DUS (WUS 1: 35.9%; WUS 2: 47.1%; WUS 3: 51.1%; WUS 4: 51.9%; WUS 5: 52.8%; DUS 1: 27.3%; DUS 2: 36.5%; DUS 3: 38.5%; DUS 4: 39.1%; DUS 5: 43.2%). Outcome in DUS still was reduced. However, with the application of advanced imaging techniques, the statistically significant difference disappeared.
Besides outcome, WUS and DUS also seem to differ in pathophysiologic characteristics. An increase in platelet aggregation as well as a blood pressure surge during awakening are said to be associated with WUS but not DUS.29,30 WUS might occur right before awakening and therefore be comparable with a stroke population with documented symptom onset.31 Indeed, it was shown that clinical and imaging characteristics in WUS and stroke eligible for intravenous thrombolysis seem to be comparable, whereas DUS—as in our cohort—tends to have a worse prognosis.13,32,33 We did not observe significant differences in baseline characteristics. Longer imaging-to-groin times in WUS and DUS can be explained by a higher percentage of MR imaging scans and might indicate a careful patient selection. An average 138 minutes in controls is attributed to many patients being secondarily transferred for mTE/aTE.10
This study has several limitations. First, due to the retrospective design, selection bias can be suspected because we do not know in which cases mTE or aTE was not considered. Also, a certain inconsistency in decision-making can be assumed. Second, the definition of WUS and DUS could lead to some overlap of groups. Some of the patients categorized as having DUS by the assessing neurologist (eg, patients arriving late in the evening) could be WUS instead. There was no common CTP protocol or quantitative mismatch measurement, which may reduce comparability. An imbalance in group size might introduce a power problem (especially in subgroup analysis).
Conclusions
Our data suggest that mTE/aTE in selected patients with WUS allows a good functional outcome comparable with that in patients treated within 6 hours of symptom onset. Patients with DUS seem to be inferior to controls regarding outcome and mortality rates. The application of advanced imaging modalities (MR imaging, CTP) significantly increases the frequency of good functional outcome in both WUS and DUS and seems to reduce mortality.
Acknowledgments
We were supported by Hiltrud Niggemann in performing the statistical analysis. Casjupea Knispel, stroke and study nurse of the Neuroradiological Clinic, participated in data collection and conducted the follow-up interviews.
Footnotes
Disclosures: Marta Aguilar Pérez—UNRELATED: Consultancy: phenox, Comments: proctor and consultant. Hansjörg Bäzner—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Bayer AG, Vital Pharma GmbH, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo. Hans Henkes—UNRELATED: Board Membership: phenox, Comments: I am Speaker of the Board without payment; phenox compensates travel expenses; Consultancy: phenox, Comments: I am a consultant phenox and receive financial compensation for this effort; Patents (Planned, Pending or Issued): phenox, Medtronic, Comments: I am a coinventor of several intellectual property claims held by Medtronic and phenox; Stock/Stock Options: phenox, Comments: I am cofounder and shareholder of phenox; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: phenox, Comments: I receive compensation for travel expenses for activities on behalf of phenox.
References
- Received August 21, 2017.
- Accepted after revision November 13, 2017.
- © 2018 by American Journal of Neuroradiology