Abstract
SUMMARY: Imaging can help to diagnose CNS vasculitis. Yet so far, no imaging studies of CNS vasculitis at 7T are available. We share our experience of vessel wall imaging (VWI) at 7T in patients with suspected vasculitis. All included patients (n=45) underwent a clinically approved 7T MRI comprising high-resolution arterial TOF angiography as well as high-resolution VWI with T1 sampling perfection with application-optimized contrast using different flip angle evolution (SPACE) and T1 SE acquired pre- and postcontrast. Twenty-three patients showed negative and 22 patients showed positive VWI at 7T. Ten of 22 7T VWI-positive cases were suggestive of vasculitis with 9 patients showing VWI of large- and medium-size vessels and 1 patient VWI of small vessels. Small-vessel vasculitis was only depicted with 7T VWI, but not 3T VWI. Our work demonstrates that diagnosing CNS vasculitis, especially small-vessel vasculitis, is feasible at 7T and highlights the potential of high-field VWI encouraging further studies in this field.
ABBREVIATIONS:
- FS
- fat-saturated
- TIC
- Translational Imaging Center
- VWI
- vessel wall imaging
CNS vasculitis is a relatively rare but serious and potentially life-threatening condition.1,2 The diagnostic process of CNS vasculitis is usually challenging, because there are currently no specific biochemical, immunologic, serologic, or imaging techniques available.3 A biopsy of the leptomeninges or cortex remains the standard, yet it is an invasive method associated with relevant morbidity and it is prone to sampling error and thus, prone to false-negative results.4,5 DSA has strongly varying sensitivity, decreasing significantly in small vessels, emphasizing that a normal angiogram therefore does not exclude primary CNS vasculitis.6⇓–8 Vessel wall imaging (VWI) is a useful adjunct technique to visualize vascular changes indicative of vasculitis that differ, for example, from intracranial atherosclerotic plaques and other causes of intracranial arterial narrowing.1,9 Current vessel wall visualization techniques at 3T are limited by its special resolution and SNR limits and do not resolve smaller intracranial vessels. Common pitfalls are caused by slow flow, dural enhancement, veins, and vasa vasorum mimicking arterial wall thickening or enhancement.9 So far VWI for the assessment of vasculitis has mainly been performed at 3T9 and explored at lower field strengths.10 While VWI at 7T is widely explored, especially in atherosclerotic disease and aneurysms, imaging studies specifically dedicated to vasculitis at 7T are currently not available.11 The aim of this work is to share our experience of VWI at 7T in patients with suspected vasculitis and highlight the potential of this technique at higher field strength.
MATERIALS AND METHODS
We retrospectively screened the health records of our tertiary care institution to identify patients examined at 7T MRI with clinical suspicion of any CNS vasculitis. This retrospective analysis was approved by the local ethic committee (2020-02902). As far as being applicable to this brief report, the Strengthening the Reporting of Observational Studies in Epidemiology guidelines were followed. Patients with suspicion of any form of CNS vasculitis were included (ie, involvement of large vessels, small vessels or mixed pattern, suspected or confirmed neurovascular pathologies). Cases with isolated leptomeningeal enhancement only were classified as negative for vessel wall enhancement.
All patients were scanned on a clinically approved 7T whole-body MRI scanner (Magnetom Terra, clinical mode, Siemens Healthcare) equipped with a 1-channel transmit and 32-channel receive head coil (Nova Medical) and on a 3T scanner (Magnetom Prisma, Siemens Healthcare) with a 32-channel head coil in the same session at the Translational Imaging Center (TIC). This retrospective analysis was approved by the local ethics committee. All patients were informed about the MRI-related risks before the examinations as a part of the routine procedure and signed a general consent form.
VWI at 7T comprised axial arterial TOF angiography 0.14 × 0.14 × 0.25 mm3 (interpolated, acquired: 0.36 × 0.28 × 0.50 mm3) with whole brain coverage; axial T1 sampling perfection with application optimized contrast by using different flip angle evolution (SPACE; Siemens) isovoxel 0.50 × 0.50 × 0.50 mm3 with TR/TE = 1200/13 ms, variable flip angle, turbo factor of 40, and total acquisition time 9 minutes and 16 seconds; as well as T1 spin echo (SE) of a selected region of interest with 0.20 × 0.20 × 1.0 mm3 and TR/TE = 700/13 ms, flip angles = 71°/135°, FOV = 195 × 240 × 16, 2 averages, and total acquisition time of 7 minutes and 45 seconds. The remaining sequences comprised: 3D MP2RAGE, SWI, and T2 TSE sequences as previously described.12
The 3T MRI followed a standardized (standard product sequences) vasculitis contrast-enhanced protocol including TOF angiography 0.50 × 0.50 × 0.50 mm3, VWI axial T1 SPACE 0.50 × 0.50 × 0.50 mm3 (extrapolated) precontrast and postcontrast, TR/TE = 600/12 ms, 2D axial T2 fat-saturated (FS) dark-blood postcontrast 2D axial T1 FS dark-blood, and 2D axial T1 SE 2.0 mm slice thickness, TR/TE = 500/13 ms, flip angle = 70°, FOV = 159 × 159 × 19.8, as well as DSC perfusion if not available from recent examinations. Macrocyclic IV contrast agent (gadobutrol 1 mmol/mL, 1 mL/10 kg body weight, 5 mL/s; Gadovist) was administered. Exemplary T1 SPACE and T1 SE images at 3T and 7T are illustrated in the Supplemental Data.
We extracted the referral information, radiologic reports, final clinical diagnosis and follow-up information if available. VWI was defined as suggestive of vasculitis with the presence of circumferential contrast enhancement in multiple foci on multiple slices with or without abnormalities on TOF angiography. The findings were regarded independent of different etiologies of vasculitis, however, the distribution pattern of vessel involvement was reported. All examinations were interpreted in the clinical routine by 2 experienced clinical readers (5 and 10 years of 7T MRI experience). No additional image reading was performed.
RESULTS
Forty-five patients (20 men and 25 women) were examined between November 2020 and July 2023. Median age was 54.8 years (mean 53.6, range 17.8–83.3). All examinations were performed without interruption or side effects. All cases referred for imaging at the TIC were ambiguous, based on standard diagnostic work-up for vasculitis including 1.5T or 3T MRI with or without prior VWI (Supplemental Data). Twenty-one patients had acute or subacute infarcts, 5 patients presented with hemorrhages, and 19 with other pathologies (chronic stroke, leptomeningeal enhancement, white matter lesions). Twenty-one of these patients underwent prior VWI in the routine clinical setting, of whom 10 had vessel wall enhancement. Specifically, the cohort includes cases with an equivocal clinical picture, discrepancies between imaging sessions, diverging multimodal evidence or mismatch between the clinical picture and cerebrovascular findings (Supplemental Data).
All examinations at the TIC were performed in the following order: 7T examination without contrast agent, 3T examination with sequences pre- and postapplication of IV contrast, and 7T examination with contrast agent. The mean time between contrast application and acquisition of T1 SPACE at 7T was 20 minutes (mean 18 minutes, minimum 6, and maximum 54 minutes; the delay was equally distributed among the VWI-negative and VWI-positive cases).
According to 7T radiologic reports, all examinations showed diagnostic quality. Twenty-three (51%) of 45 cases were reported as negative, including 1 previously VWI-positive case in clinical routine at 3T (Supplemental Data) based on negative VWI sequences on 7T MRI with subsequent exclusion of vasculitis. Twenty-two (49%) of 45 cases were reported with positive VWI findings. Ten of those 22 cases were suggestive of vasculitis, 9 affecting large and midsize arteries (Fig 1) and 1 exclusively affecting perforating arteries that are considered to be small vessels, as their diameter is smaller than 300 µm (Fig 2, Supplemental Data).
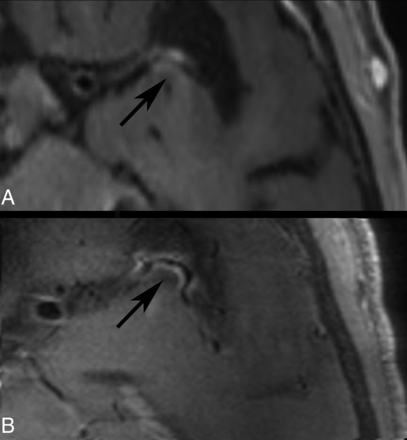
Medium- to large-vessel vasculitis. Representative T1 SPACE images of a patient later diagnosed with CNS vasculitis due to Borrelia burgdorferi infection are presented at admission at 3T (A) and 7T (B). VWI at 3T and 7T show enhancement of the proximal M2 segment. The thickened, enhancing vessel wall is more clearly delineated at 7T compared with 3T.
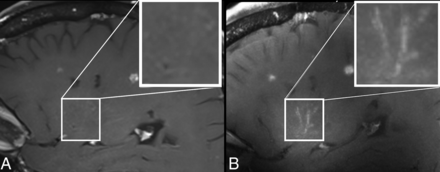
Detection of small vessel vasculitis at 7T with VWI in a patient with Sjögren disease and subacute stroke. Sagittal T1 SPACE contrast-enhanced vessel wall images are shown at 3T (A) and 7T (B). The MCA perforators are enlarged in both images. At 7T, vessel wall enhancement of the MCA perforators, not visible at 3T, is visualized. The MCA perforators are enlarged in both images to better appreciate vessel wall enhancement at 7T and a very faint perforator vessel silhouette at 3T in knowledge of the 7T images. Medium-sized vessels were not affected in this patient.
The remaining 12 cases were reported with arteriosclerosis (7), nonvasculitic small vessel disease (2), dolichoectasia (1), local inflammatory process not related to vasculitis (1), and persisting imaging changes after inflammation (1).
DISCUSSION
In this report, we highlight the potential of 7T imaging to support the diagnosis of CNS vasculitis, because it overcomes several limitations encountered at lower field strengths.
In contrast to 3T, 7T has a higher SNR and imaging resolution and is able to sharply resolve the vessel wall and its lumen.13 We used a whole-brain 3D T1 SPACE sequence and a 2D T1 TSE sequence, covering the brain area adapted to the clinical question. As illustrated in this brief report, vasculitic vessel wall enhancement can be better delineated at 7T with both sequences compared with 3T. T1 TSE achieved a higher in-plane resolution allowing slightly better characterization of a particular vessel segment. However, the whole brain coverage of T1 SPACE is advantageous, because vasculitis often affects several vessel segments.14 Also, the possibility to assess the whole vessel wall circumference with 3D isotropic data of T1 SPACE improves analysis. Importantly, smaller vessels, such as perforating arteries, are robustly visualized at 7T with the whole-brain T1 SPACE. This is of particular significance, as other techniques such as DSA are only moderately sensitive in detecting VWI of small vessels.
Thus, VWI at 7T may help to depict especially deep small vessel involvement in, for example, autoimmune connective tissue disorders that are prone to cerebrovascular events compared with the overall population. To date, there are only a few published case reports about VWI in patients with rheumatic diseases. For example, a published case report of CNS vasculitis in a patient with systemic lupus erythematosus who initially presented with stroke, showed circular VWI of the distal ICA and the M1 segment, consistent with vasculitis.15 Another case report of a patient with Sjögren disease with stroke showed circular VWI in the ICA, M1, and the A1 segment, also consistent with CNS vasculitis.16 In the patient with Sjögren disease in our cohort, only perforating vessels were affected, but no medium-sized vessels. Because detection of vessel wall enhancement of perforating vessels is only feasible at very high field strength, we hypothesize that CNS vasculitis is likely underdiagnosed in patients with rheumatic diseases, and merits further investigations.
Also for disease monitoring, 7T VWI may add value because it allows better visualization of the vessel wall and consequently treatment effects. Importantly, as evident in our patient collective, the presented imaging technique is feasible in clinical routine and can help to solve ambiguous cases, with the increasing availability of FDA-approved and Conformité Européenne-certified 7T MR scanners.
Our work has limitations, as the time of contrast application and the acquisition of VWI sequences is nonstandardized, not established at 7T (in contrast to lower field strength17), and varied between individuals but to a similar degree in VWI-positive and VWI-negative cases in this study. Nevertheless, quantitative analysis of vessel wall enhancement was not feasible due to different contrast delay time and a small patient collective. Properties of contrast enhancement as well as optimal delay for VWI at 7T including direct application at 7T need to be further studied; they have been examined to some extent, however, for other applications but not specifically for CNS vasculitis.18,19 Potentially, delayed imaging could lead to a decrease of false-positive findings reducing specificity. On the other hand, sensitivity could increase, because the contrast agent has more time to accumulate within the vessel wall. In the field of CNS vasculitis specifically, a prospective head-to-head comparison of 7T versus 3T would be necessary to prove a benefit of the higher field strength. Furthermore, advantages and pitfalls of T1 SPACE and T1 SE at 7T should be examined with T1 SPACE providing whole brain coverage and T1 SE providing superior SNR in a selected field of view. Finally, subgroups of patients with CNS vasculitis benefiting most from 7T must be identified. In summary, higher resolution VWI at 7T is feasible, addresses several limitations encountered at lower field strength, and shows the potential to improve detection of CNS vasculitis, including involvement of small vessels, encouraging further studies in this field.
Acknowledgments
We kindly thank our magnetic resonance imaging technologists, in particular Samuel Stettler, Sabrina Herzog, and Osman Limanoski for the excellent support.
Footnotes
Indicates article with online supplemental data.
This research has been supported by the sitem-insel Support Funds 2021.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received July 3, 2024.
- Accepted after revision December 3, 2024.
- © 2025 by American Journal of Neuroradiology