Abstract
BACKGROUND AND PURPOSE: Aneurysmal disease of the intracranial vasculature poses a relevant threat, warranting effective interventions. Minimally invasive interventional techniques for aneurysm treatment have evolved to the application of flow-diversion stents and devices. This study focuses on the Contour Neurovascular System (CNS), aiming to add knowledge regarding its mid- to long-term outcomes in treating wide-necked intracranial aneurysms.
MATERIALS AND METHODS: Conducted in accordance with STROBE guidelines, this study retrospectively evaluated all patients with intracranial aneurysms treated with CNS embolization. Demographic and interventional data were collected retrospectively, including aneurysm characteristics, procedural details, and angiographic follow-up evaluations up to 24 months after CNS implantation.
RESULTS: A total of 106 patients with 109 aneurysms were included in this study, whereby 72 patients were treated for an incidental aneurysm, while 34 patients presented with subarachnoid hemorrhage. Implantation was successful in 95.5% of patients. Occlusion rates were as follows: 6 months (69/106, 65.1%): Raymond-Roy-Scale (RRS) 1 44/69 (63.4%), RRS 2 16/69 (23.1%), RRS 3a 4/69 (5.8%), RRS 3b 5/69 (7.2%); 12 months (44/106, 41.5%): RRS 1 24/44 (55.5%), RRS 2 12/44 (27.3%), RRS 3a 4/44 (9.0%), RRS 3b 4/44 (9.0%); 24 months (30/106, 28.3%): RRS 1 21/30 (70.0%), RRS 2 8/30 (26.7%), RRS 3b 1/30 (3.3%). Periprocedural complications: Overall 8/106 (7.5%); elective cases 4/72 (5.5%); aneurysm rupture 4/34 (11.7%). Adjunctive devices were used in 13/106 cases (12.2%).
CONCLUSIONS: The present work reports the long-term angiographic and clinical follow-up results of a single-center cohort of 106 patients with intracranial aneurysms treated with the CNS. The CNS demonstrated a high rate of successful implantation and promising mid- and long-term stability, with a low reintervention rate beyond 24 months in patients exhibiting early occlusion at 6 months. While acknowledging the limitations, these findings contribute valuable information about the safety and efficacy of the CNS, and warrant continued exploration in larger, prospective studies to validate its role in aneurysm treatment.
ABBREVIATIONS:
- AcomA
- anterior communicating artery
- CNS
- Contour Neurovascular System
- DTN
- dome-to-neck ratio
- RRS
- Raymond-Roy Scale
- WEB
- Woven EndoBridge
Aneurysmal disease of the intracranial vasculature represents a potentially disabling and life-threatening condition. Over the past few decades, various minimally invasive techniques for interventional aneurysm treatment have been conceived and established. In recent years, treatment options have expanded beyond conventional balloon- and stent-assisted aneurysm coiling to implanting flow-diversion stents and intrasaccular flow-disruption devices. These stents and devices do not immediately occlude the aneurysm; instead, they mitigate or disrupt the intra-aneurysmal blood flow to effectively reduce the mechanical stress on the aneurysmal wall to prevent rupture. Furthermore, turbulences in intra-aneurysmal flow promote growth of a neointimal lining across the aneurysm neck and ultimately propagate occlusion of the aneurysm.1,2 The described effects of flow-diversion can be achieved with flow-diversion stents; however, this is only possible when compromising the parent vessel.3 In addition to stent placement, the Woven EndoBridge (WEB Device, Terumo Microvention) represents a well-studied intrasaccular flow-disruption device. In 2021, the novel Contour Neurovascular System (CNS; Stryker) was introduced as the then-newest development in intrasaccular flow-disruption devices intended for minimally invasive interventional treatment of wide-necked intracranial aneurysms.4,5 The CNS has been the subject of multiple research efforts and although limited to mostly retrospective studies, it has shown good results for aneurysmal occlusion, peri-interventional complications, and short-term stability.4,6⇓-8 Research results on long-term aneurysmal occlusion after CNS implantation are not available at this date.
The present work reports the results of the currently available largest single-center cohort of patients with intracranial aneurysms treated with the CNS.
MATERIALS AND METHODS
This study was approved by the ethics committee of the Christian Albrechts University in Kiel and was conducted in accordance with the STROBE guidelines and the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Contour Neurovascular System Implantation Procedures
Decisions to implant the CNS were made in consensus with the patients, their relatives, and the hospital’s neurosurgical department after careful review of the clinical condition and preoperative imaging studies. The CNS was implanted under general anesthesia in all cases. Interventions were performed by 1 of 5 board-certified interventional neuroradiologists (F.W., S.P., N.L., J.H., O.J.) in a biplanar angio suite. A triaxial setup with a 90-cm sheath (Neuron Max, Penumbra, or Cerebase, Cerenovus) and an intermediate catheter (Sofia 5F or EX, Microvention) was used. For probing the aneurysm and positioning and placement of the CNS, we used 0.021-inch and 0.027-inch microcatheters, depending on the size of the CNS (Headway 27, Microvention; Phenom 27, Medtronic; XT 27, Stryker; VIA 27, Microvention). The use of further devices was left to the interventionalists’ discretion.
In elective cases, preoperative DSAs were acquired to determine the feasibility and method of interventional treatment. The CNS was sized during the implantation procedures by the acquisition of 3D angiography conebeam CT imaging, as it provided the best possible anatomic representation of the aneurysms and allowed for multiplanar visualization. Device placement and deployment were completed in a designated working projection, in which the aneurysm and especially its neck could be visualized in the best way possible.
Population
All patients who had received intracranial aneurysm treatment with implantation of a CNS at our center were retrospectively included in this study. Demographic data were collected by extensive patient chart review. Interventional and cross-sectional imaging findings were collected retrospectively between 2018 and 2023. Clinical follow-up findings and neurologic conditions were assessed by thoroughly reviewing the in- and outpatient documentation. Baseline demographic characteristics were noted.
Aneurysm Characteristics and Interventional Data
We report the number of aneurysms, including aneurysm type (bifurcation or sidewall), morphology (saccular or lobulated), neck and dome width, height, dome-to-neck ratio (DTN), affected vascular territory, parent vessel diameter, rupture status, and whether prior interventional treatment efforts had been conducted.
Moreover, mean total intervention times, dose-area product in cGy/cm2, and modified Raymond-Roy Scale (RRS) values at the end of the procedure were noted. The case-specific CNS time was noted, describing the timeframe between the first angiogram in the best possible anatomic plane and CNS detachment.
Clinical and Imaging Follow-Up and Follow-Up Evaluation
Follow-up imaging was conducted with DSA, CTA, and MRI. In elective patients, CT angiography focused on the device, and MRI examinations were conducted within 24 hours of the intervention. Independent of rupture status, DSA, CTA, and, where appropriate, MRI were scheduled at 6 months after CNS implantation and followed by yearly noninvasive controls with CTA or MRI. Further cross-sectional imaging results at 1 and 2 years after the procedure and beyond were collected if available. Results of the latest available angiographic follow-up imaging were noted.
Any peri-interventional complications that occurred within 24 hours of the procedure and cases in which CNS implantation was considered unfeasible were noted. We further divided complications into procedure- and device-related complications.
The final procedural images and angiographically assessable follow-up images (DSA, CTA) were evaluated by using the modified RRS to determine the aneurysmal occlusion status.9,10 Adequate occlusion was defined as full occlusion of the complete aneurysm and presence of a neck remnant (RRS 1, 2). In terms of clinical follow-up, baseline NIHSS and mRS values were retrospectively collected.11,12 Both score values were noted at various points in the postinterventional follow-up in combination with imaging follow-up results. In the setting of aneurysmal hemorrhage, the Hunt and Hess scores were documented.13,14
RESULTS
Patients and Aneurysms
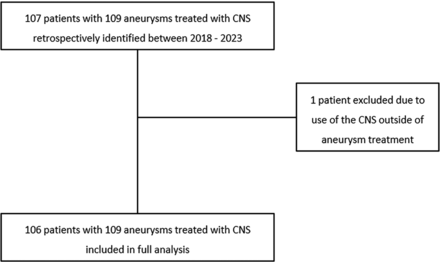
In the study’s timeframe between 2018 and 2023, 106 patients with 109 aneurysms were retrospectively analyzed from our prospective CNS database. The patient inclusion flowchart is depicted in Fig 1. Median age (range) was 68 years (27–88). In terms of sex, there were 72 women and 34 men. Demographics are summarized in Table 1. Seventy-two patients were treated because of incidental aneurysms, while 34 patients were treated in the framework of aneurysmal rupture with subarachnoid hemorrhage. In 4 cases, retreatment after previous surgery or embolization (1 case of clipping, 1 case of WEB, and 2 cases of previous coiling) was performed. One case was excluded because of off-label use of the CNS in a situation outside of aneurysmal treatment. Further aneurysmal characteristics are summarized in Table 2.
Patient selection flow chart.
Demographics
Aneurysm localization and periprocedural characteristics
Findings from specific subgroups of the present analysis were previously published in 2 research studies conducted at our institution.15,16
Of the 109 aneurysms, 88 were located at a bifurcation, while 21 were sidewall aneurysms. Overall, 89 aneurysms were located in the anterior circulation. Here, 26 aneurysms were localized in the anterior communicating artery (AcomA), 22 in the ICA (of which 9 were at the carotid bifurcation [ICA-T] and 13 along the sidewall of the terminal ICA), 30 at the bifurcation of the MCA, 7 at the posterior communicating artery (of which 5 were at a bifurcation and 2 sidewall aneurysms), and 2 in the pericallosal artery.
Of the 20 aneurysms in the posterior circulation, 17 were located at the basilar tip, 1 in the posterior communicating artery, and 2 in the posterior inferior cerebellar artery.
Mean aneurysm neck size was 3.9 mm (range 1.7–11.6 mm). Mean dome size was 6.7 mm (range 2.5–24.2 mm). Mean aneurysm height was 7.6 mm (range 2.4–26.1 mm). Mean DTN was 1.7 (range 0.93–3.51). Sixty-five aneurysms were considered to have a wide neck with a DTN <1.6.
Among the 106 cases, we found a mean full procedure time of 78 (range 23–209) minutes with a specific Contour time of 33 (range 44–172) minutes. Mean radiation dose of the full procedures was 7886.3 (range 277–197,177) cGy/cm2.
Peri- and Postinterventional Antiplatelet Management
In elective cases, patients received a single-dose of aspirin 100 mg and clopidogrel 75 mg before the intervention. After completion of the embolization procedure, aspirin 100 mg was continued for another 4 weeks and then stopped, except for cases where it was continued for nonaneurysm-related reasons.
In the framework of aneurysm rupture, antiplatelet management depended on the occlusion status of the aneurysm. In the case of visual flow-disruption without thrombus formation, no antiplatelet medication was administered. In case of beginning occlusion of the aneurysm or apparent flow disruption, 500 mg of aspirin IV was administered as a single-shot, and then 100 mg of aspirin daily was continued for 4 weeks. In case of instant aneurysm occlusion or excessive thrombus formation, intravenous tirofiban was administered adapted to body weight.
Postinterventional Follow-Up before Discharge
Per our protocol, patients treated in an elective setting were scheduled for postinterventional cranial MRI and CTA. In patients who suffered subarachnoid hemorrhage, imaging follow-up did not follow a specific protocol within 24 hours.
Within 24 hours, 79/106 (74.5%) patients received angiographically assessable imaging, whereby 3 received DSA while 76 received CTA. Here, 54 patients (68.4%) were graded as RRS 1/2.
A total number of 63/106 (59.4%) received an MRI within 24 hours, whereby 38 patients were seen to have spotty DWI lesions. Among these patients, we found a mean number of 4 DWI lesions (range 1–12 lesions).
Follow-Up Results after 6 Months
Follow-up results are displayed in Table 3. For every patient, an appointment for DSA or CTA and MRI was scheduled 6 months after CNS implantation. A total of 69/106 (65%) received angiographically assessable imaging, including 55 patients after elective treatment and 14 patients after subarachnoid hemorrhage. Here, 12 patients received DSA, 10 received CTA, 27 received DSA and CTA, and 20 received CTA and MRI. Upon imaging, 60 patients (86.9%) were graded as RRS 1/2. Four patients (6.6%) were graded as RRS 3a and 5 (8.3%) as RRS 3b. Within 6 months, 5 patients underwent retreatment. Out of the 60 patients rated as RRS 1/2, 29 did not receive any further follow-up.
Follow-up results after 6, 12, and 24 months
Follow-Up Results after 12 Months
At 12 months after CNS implantation, angiographically assessable imaging follow-up is available for 44/106 patients (41.5%). Of these patients, 6 received DSA, 20 received CTA, 2 received DSA and CTA, and 16 received CTA and MRI. Initially, 36 were treated in an elective setting, while 8 presented with aneurysm rupture. Here, 36 aneurysms (81.8%) showed an adequate occlusion, 10 after rupture and 26 after elective treatment. Four patients were rated as RRS 3b, whereby 1 was after rupture. Four patients after elective treatment were rated as 3a. One of the patients rated as RRS3b had been retreated.
Follow-Up Results after 24 Months
Angiographic follow-up 24 months after the procedure is available for 30/106 patients (28.3%), 22 after elective treatment, and 8 after aneurysm rupture. Overall, 1 patient received DSA; 1 patient received DSA, CTA, and MRI; 21 patients received CTA; and 7 patients received CTA and MRI. Here, adequate occlusion was seen in 29 patients (96.6%). One patient who initially presented with aneurysm rupture was rated as RRS 3b and subsequently underwent retreatment.
Latest Available Follow-Up beyond 6 Months
The latest available follow-up beyond 6 months was available for 76/106 patients (71.6%, Table 4). Overall, the median angiographic follow-up was 16.6 months (interquartile range [IQR] 8.2–22.0). At the latest follow-up, 69 patients (90.7%) were seen to be adequately occlusion while 3 patients (3.9%) were graded as RRS 3a and 4 (5.2%) as RRS 3b.
Latest available follow-up results
Reinterventions
A reintervention was performed in 7/106 cases (6.6%). Here, 5 reinterventions were performed within the first 6 months after CNS implantation due to early recurrences. Two late reinterventions were performed after 12 months and 24 months, respectively.
Of the 7 cases that underwent retreatment, 4 patients initially presented with aneurysm rupture. Three aneurysms were located in the distal internal carotid artery, 1 aneurysm in the basilar tip, 2 in the posterior communicating artery, and 1 in the posterior inferior cerebellar artery. They included 4 sidewall and 3 bifurcation aneurysms. In 2 cases, additional coiling was performed for optimal aneurysm occlusion. These results are summarized in Table 5.
Characteristics of nonoccluded aneurysms
The patient undergoing late reintervention after 12 months had a large ICA aneurysm (5.4 × 13.8 × 14.9 mm; DTN 2.56) that had been primarily treated with CNS implantation in combination with large-volume coiling (CoCoJaMBO). Upon the first follow-up after 12 months, prolapse of both the CNS and coil packet into the aneurysm was evident, which required placement of a flow-diversion stent. Follow-up data after 6 months was not available for this patient.
The patient undergoing late reintervention after 24 months was known to have remaining perfusion of the aneurysmal lumen (RRS 3b) at the 6-month follow-up appointment that was primarily managed with a “watch-and-wait” strategy. Over the course of the 1- and 2-year follow-up, progressive reperfusion was seen, setting the indication for retreatment with a flow-diversion stent.
Complications, Device Failures, and Deaths
In total, a periprocedural complication was found in 8/106 cases (7.5%). Among the 72 patients treated in an elective setting, a complication developed in 4 patients (5.5%). In 2 patients, iatrogenic dissection of the internal carotid artery was seen, which remained without clinical sequelae. One patient developed aphasia after the intervention, which resolved after intravenous thrombolysis, and 1 patient developed light hemiparesis due to basal ganglia infarction, which resolved after 48 hours.
In acute-setting patients, a complication was reported in 4/34 (11.7%) cases. A total of 3 thromboembolic events occurred. In 1 case, acute bleeding from the aneurysm dome was seen after deploying the CNS, requiring additional coiling.
A technical complication was documented in 1 case (0.9%). Here, the CNS accidentally detached after the device was pushed out of the microcatheter. The CNS remained inside the aneurysm dome while the base was still well-perfused. To completely occlude the aneurysm, another CNS with additional coiling (CoCoJaMBO) was placed.
In 5 patients, CNS implantation was intended but not performed as planned. Here, in the case of an ICA aneurysm, the CNS could not be positioned without affecting the origin of the ophthalmic artery, leading to placement of a WEB. In another case of an MCA bifurcation aneurysm, 2 sizes of the CNS could not achieve adequate occlusion, hence, embolization was completed with WEB. In another case of an MCA bifurcation aneurysm, the CNS could not be placed in such a manner to achieve sufficient flow disruption. Thus, embolization was done by coiling. In a case of an AcomA aneurysm, the CNS caused flow-impairment of the A2, and therefore, aneurysmal coiling was performed. In 1 case, both CNS and WEB placement were deemed insufficient. In this patient, flow-diversion stent placement of the parent vessel was needed.
Adjunctive devices were used in 13 cases (12.2%), whereby additional coiling was done in all cases.
Five patients died, whereby 4 deaths occurred as a result of aneurysmal rupture and subarachnoid hemorrhage. Two patients died following the sequelae of severe subarachnoid hemorrhage. One patient experienced fulminant pulmonary embolism 10 days after the procedure. Another patient died because of respiratory insufficiency. One patient treated in an elective setting died after 2 months because of an underlying abdominal tumor.
DISCUSSION
This retrospective single-center analysis reports the results after the embolization of 109 aneurysms by using the novel CNS. To date, our study represents the largest single-center cohort investigated regarding the use of this novel device for intracranial aneurysm embolization.
The main findings of our study are 1) CNS implantation was completed successfully in 95.5% (104/109) of the aneurysms as planned.; 2) follow-up imaging examinations show adequate aneurysmal occlusion in 89.5% after 6 months, 87.5% after 1 year, and 94.1% after 2 years; 3) overall, a reintervention was necessary in 5.6% while no reintervention was reported after 24 months if adequate occlusion was confirmed after 6 months; 4) a periprocedural complication was found in 7.5% of the cases.
Given its design and disc-shaped appearance, the CNS places itself inside the aneurysm such that a maximum attenuation of nitinol wires is achieved at the level of the aneurysm neck, promoting flow disruption, neointimal growth, and, thus, progressive thrombosis of the aneurysm.2,17
Successful Device Implantation and Procedural Considerations
The CNS is intended for treatment of wide-necked bifurcation aneurysms; studies have shown good applicability of this device in different aneurysm types in different locations. In the present study, the CNS was implanted as planned in 95.5% of the cases. Overall, 61% of aneurysms were wide-necked and 39% were narrow-necked and located in different localizations and configurations; hence, a primary implantation rate of 95.5% underscores the broad applicability of the CNS. In addition, the rates of 9.4% and 7–14% use of adjunctive devices during WEB implantation reported by Popielski et al18 and Lv et al19 are in good agreement with our reported rate of 12.2%.
Follow-Up Results
In our study, we found an adequate occlusion rate (RRS 1/2) in 89.5% of patients after 6 months, 87.5% after 12 months, and 94.1% after 24 months. Occlusion status was assessed on DSA or CTA imaging, while MRI does not allow for RRS grading due to artifacts of the device. Exemplary cases are shown in Figs 2 and 3. Currently, the largest studies investigating patients treated with the CNS are the Contour Neurovascular System - European Pre-Market Unruptured Aneurysm (CERUS) study6 in 2022, Biondi et al7 in 2023, and Griessenauer et al20 in 2024. The CERUS study,6 prospectively investigating 34 patients, showed an adequate occlusion rate of 77% after 6 months and 90% after 12 months, while Biondi et al7 report an adequate occlusion rate of 81.4% after 3 months and 89.4% after 12 months in 60 elective aneurysms. Griessenauer et al20 report 91.5% adequate occlusion after 12 months, which is in good agreement with our reported rate of 90.8% at latest follow-up. Since 2019, studies on the CNS have reported occlusion rates varying between 80–100% in follow-up timeframes up to 1 year, whereby some studies only cover a small number of patients and some only CNS-assisted procedures.4⇓⇓⇓-8,21⇓-23 These studies are included in a meta-analysis published by Ghozy et al24 in 2024 including 131 aneurysms and an updated meta-analysis by Jagtiani et al25 including 206 aneurysms with a pooled adequate occlusion rate of 85%. As for the WEB device, 2 meta-analyses covering 588 and 967 aneurysms determined a midterm adequate occlusion rate of 85% and 81%, respectively, which is slightly lower than our findings.19,26 Overall, our study further confirms the high rates of adequate occlusion that can be achieved when using the CNS for aneurysm embolization.
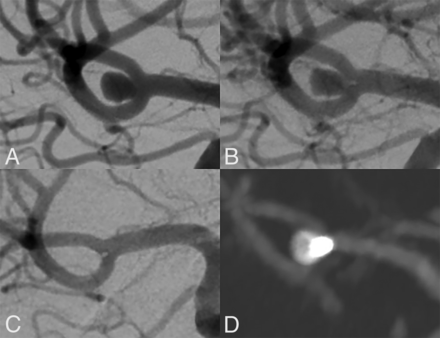
Long-term stability of a wide-necked MCA bifurcation aneurysm treated successfully with CNS implantation. This wide-necked MCA bifurcation aneurysm (A, dome-to-neck ratio 1.32) was treated electively with implantation of a 5-mm CNS. At the end of the procedure, residual perfusion was still present (B). In follow-up DSA after 6 months and CTA after 24 months, the aneurysm is seen to be fully occluded (C and D).
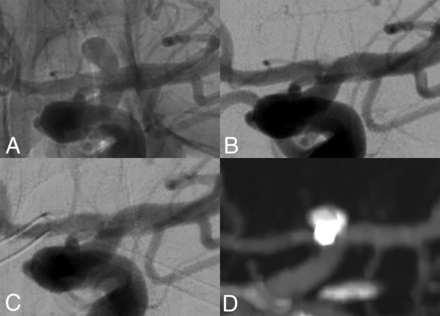
Long-term occlusion of an ICA tip aneurysm treated with CNS implantation. This wide-necked ICA tip aneurysm (A, dome-to-neck ratio 1.6) was successfully embolized with a 5-mm CNS. At the end of the procedure, beginning aneurysmal thrombosis was seen (B). DSA after 6 months and CTA after 24 months confirmed stable occlusion (C and D).
Technical and Procedural Complications and Reinterventions
Overall, a total of 8 (7.5%) periprocedural complications occurred in our cohort, including 3 thromboembolic events (2.8%), 2 dissections (1.9%), and 1 intraprocedural bleeding (0.9%) (Table 6). This is in good agreement with the meta-analysis of Jagtiani et al25 from 2024, reporting a rate of thromboembolic events of 6%, and with Griessenauer et al20 reporting 7.2%. In comparison, Biondi et al7 reported a 6.7% rate of periprocedural thromboembolism in 60 aneurysms, while the CERUS study6 reports 11% in 34 aneurysms. However, it is noteworthy to mention that 50% of the complications in our cohort occurred in patients treated in the framework of subarachnoid hemorrhage. Additionally, all complications in the patients treated in an elective setting remained without clinical sequelae. In a meta-analysis, the thromboembolic rate of the WEB device was shown to be 5.6% in 936 patients, while a recent retrospective study reported a rate of up to 13% for thromboembolic events by using the WEB.27,28
Periprocedural complications
The rate of reinterventions in our cohort was 6.6% overall, whereby almost three-quarters of reinterventions were performed within the first 6 months after implantation. In our data, we could not identify specific risk factors for a reintervention (Table 3). However, we suspect imperfect sizing or device placement to be the most likely reason for nonocclusion. Delayed reintervention was performed in 2 patients—noteworthy here is that in 1 patient, reperfusion was already present after 6 months and was managed with a watch-and-wait approach, while in the other patient, the earliest follow-up was conducted after 12 months showing reperfusion in need of retreatment (Fig 4). Furthermore, we found that the reintervention rate for patients rated as RRS 1/2 at the 6-month follow-up appointment was 0% after 24 months, supporting the hypothesis that early occlusion after 6 months can serve as a marker for long-term stability and that the rate of rerupture after successful embolization is very low.29,30 While Biondi et al reported a 0% reintervention rate within 12 months in their cohort, other studies report reintervention rates up to 6% for aneurysms treated with the CNS in combination with adjunctive devices.6,7,21 In contrast to our findings, Griessenauer et al20 report retreatment in 2.5% out of 279 cases, which may be attributed to the significantly lower number of patients treated in the framework of subarachnoid hemorrhage compared with our cohort (16.8% versus 31.2%). Two large meta-analyses on the WEB device report a 6% and 8% rate of retreatment, respectively, which is comparable to our findings.19,27
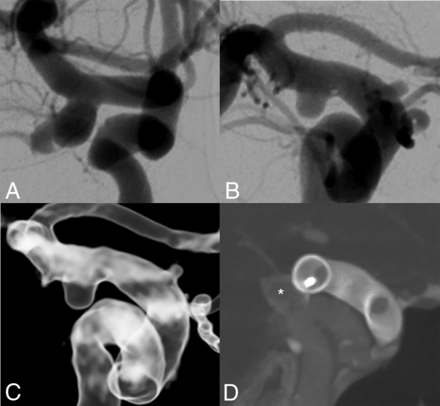
Reperfusion of an ICA aneurysm after CNS implantation treated with placement of a flow-diversion stent. In this patient, a wide-necked aneurysm (dome-to-neck ratio 1.6) located in the posterior wall of the ICA was found and treated with placement of a 9-mm CNS (A). After 6 months, reperfusion on the posterior side of the aneurysm was seen, which was primarily managed with a watch-and-wait strategy (B and C). After 12 months, the reperfusion was seen to be progressive, and a flow-diversion stent was placed over the CNS (star, D), ensuring complete aneurysmal sealing.
Limitations
One of the primary limitations of this study, besides its retrospective design, is the irregularity in follow-up appointments among participants. While patients are scheduled for follow-up before discharge and at 3, 6, 12, and 48 months, adhering to clinical standards, the absence of follow-up imaging may affect the precision and reliability of our findings. Furthermore, this study encompasses a diverse range of aneurysm types with inherent heterogeneity, which may also limit the ability to identify specific trends or patterns associated with particular aneurysm subtypes, which poses an interesting subject for further research.
CONCLUSIONS
The CNS is an effective and safe intrasaccular flow-disruption device for the treatment of aneurysms of the intracranial vasculature, showing comparable rates of occlusion, reinterventions, and peri-interventional complications to established intrasaccular treatment options. Furthermore, this study shows promising results for long-term stability of the CNS.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received July 5, 2024.
- Accepted after revision September 26, 2024.
- © 2025 by American Journal of Neuroradiology