Abstract
BACKGROUND AND PURPOSE: Systemic lupus erythematosus is a complex autoimmune disease known for its diverse clinical manifestations, including neuropsychiatric systemic lupus erythematosus, which impacts a patient’s quality of life. Our aim was to explore the relationships among brain MR imaging morphometric findings, neuropsychiatric events, and laboratory values in patients with systemic lupus erythematosus, shedding light on potential volumetric biomarkers and diagnostic indicators for neuropsychiatric systemic lupus erythematosus.
MATERIALS AND METHODS: Twenty-seven patients with systemic lupus erythematosus (14 with neuropsychiatric systemic lupus erythematosus, 13 with systemic lupus erythematosus), 24 women and 3 men (average age, 43 years, ranging from 21 to 62 years) were included in this cross-sectional study, along with 10 neuropsychiatric patients as controls. An MR imaging morphometric analysis, with the VolBrain online platform, to quantitatively assess brain structural features and their differences between patients with neuropsychiatric systemic lupus erythematosus and systemic lupus erythematosus, was performed. Correlations and differences between MR imaging morphometric findings and laboratory values, including disease activity scores, such as the Systemic Lupus Erythematosus Disease Activity Index and the Systemic Lupus International Collaborating Clinics Damage Index, were explored. An ordinary least squares regression analysis further explored the Systemic Lupus Erythematosus Disease Activity Index and Systemic Lupus International Collaborating Clinics Damage Index relationship with MR imaging features.
RESULTS: For neuropsychiatric systemic lupus erythematosus and non-neuropsychiatric systemic lupus erythematosus, the brain regions with the largest difference in volumetric measurements were the insular central operculum volume (P value = .003) and the occipital cortex thickness (P = .003), which were lower in neuropsychiatric systemic lupus erythematosus. The partial correlation analysis showed that the most correlated morphometric features with neuropsychiatric systemic lupus erythematosus were subcallosal area thickness asymmetry (P < .001) and temporal pole thickness asymmetry (P = .011). The ordinary least squares regression analysis yielded an R2 of 0.725 for the Systemic Lupus Erythematosus Disease Activity Index score, with calcarine cortex volume as a significant predictor, and an R2 of 0.715 for the Systemic Lupus International Collaborating Clinics Damage Index score, with medial postcentral gyrus volume as a significant predictor.
CONCLUSIONS: The MR imaging volumetric analysis, along with the correlation study and the ordinary least squares regression analysis, revealed significant differences in brain regions and their characteristics between patients with neuropsychiatric systemic lupus erythematosus and those with systemic lupus erythematosus, as well as between patients with different Systemic Lupus Erythematosus Disease Activity Index and Systemic Lupus International Collaborating Clinics Damage Index scores.
ABBREVIATIONS:
- Ang
- angular gyrus
- APS
- antiphospholipid syndrome
- C3
- complement component 3
- C4
- complement component 4
- Calc
- calcarine cortex
- norm
- normalized
- NP
- neuropsychiatric
- NPSLE
- neuropsychiatric systemic lupus erythematosus
- OCP
- occipital cortex
- OLS
- ordinary least squares
- PGA
- Physician Global Assessment
- SCA
- subcallosal area
- SLE
- systemic lupus erythematosus
- SLEDAI
- Systemic Lupus Erythematosus Disease Activity Index
- SLICC-DI
- The Systemic Lupus Erythematosus International Collaborating Clinic Damage Index/American College of Rheumatology Damage Index
- TMP
- temporal pole
- WMH
- white matter hyperintensities
Systemic lupus erythematosus (SLE) is a complex autoimmune disease, characterized by a dysregulated immune system that leads to chronic inflammation and the potential for multiorgan involvement,1 affecting 5.14 (range, 1.4–15.13) per 100,000 person-years and 0.40 million annually, respectively.2,3 SLE more frequently affects the musculoskeletal system and skin, but it can also manifest as neuropsychiatric events,4 causing significant mortality and morbidity, accounting for up to 19% of deaths in patients with SLE,5 while impairing patients’ quality of life. The etiology of neuropsychiatric SLE (NPSLE) remains poorly understood, and the identification of reliable biomarkers would be crucial for early diagnosis, prognosis, and therapeutic intervention.6
Advances in medical imaging techniques, particularly MR imaging of the brain, have provided valuable insights into the structural and functional alterations associated with NPSLE.7⇓⇓⇓-11 MR imaging allows noninvasive visualization of brain structures and facilitates the detection of abnormalities that may contribute to the development of neuropsychiatric symptoms in patients with lupus. Despite imaging advancements, there is still little consensus on the association of MR imaging measurements and the clinical side of NPSLE.
The diversity of neuropsychiatric manifestations in lupus presents significant diagnostic challenges. These manifestations can range from mild cognitive dysfunction to severe disorders, including psychosis, mood disorders, seizures, and cerebrovascular events.12⇓-14 Moreover, these symptoms may be unrelated to SLE and represent primary psychiatric conditions in the overlap with SLE, making accurate diagnosis, the attribution process, and appropriate management essential.13 Laboratory investigations, including autoantibody profiles and serologic markers, play a crucial role in the diagnosis and monitoring of lupus.
The association between structural MR imaging alterations and SLE has been investigated by few studies, such those included in the meta-analysis by Cox et al,15 in which they confirmed that hippocampus, corpus callosum, and total gray matter volume measures in patients with SLE were considerably lower than those in age- and sex-matched controls. However, their association with brain MR imaging findings and neuropsychiatric events remains insufficiently explored.
The aim of the study was to explore the correlations among brain morphometric MR imaging findings, neuropsychiatric events, and laboratory values in NPSLE.
MATERIALS AND METHODS
The study was designed as exploratory and cross-sectional. Patients with SLE were recruited from those referred to the Lupus Clinic, rheumatology department of our university hospital (Rheumatology Unit, Azienda Ospedaliero-Universitaria, Cagliari, Italy) between April 2019 and February 2020, on the basis of the following inclusion criteria: 1) fulfillment of the 2019 criteria of the American College of Radiology/European Alliance of Associations for Rheumatology; 2) 18 years of age or older; and 3) the ability to provide informed consent. The local Independent Ethics Committee examined and approved the study protocol (protocol No. PG/2019/4522). All participants provided written informed consent.
Clinical and Immunologic Data
Demographics, classic atherogenic risk variables (such as hypertension, hyperlipidemia, and smoking), and basic laboratory data were evaluated for patients and controls. Patients were classified regarding SLE duration, activity, damage, conventional serology, autoantibodies, and therapy. Disease activity by the Physician Global Assessment (PGA) was evaluated on a 0–3 visual scale.16 The SLE Disease Activity Index (SLEDAI)17 was used to evaluate the state of the disease in 9 organ systems, including the CNS, vascular, renal, musculoskeletal, serosal, cutaneous, immunologic, constitutional, and hematologic systems. An SLEDAI of ≥6 is consistent with active disease. To evaluate accumulated damage in multiple organs, we used the Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index.18
Complement component 3 and 4 (C3 and C4) levels, anti-double stranded DNA, anti-Ro/SSA, anti-La/SSB, anti-Sm, anti-RNP, and antiphospholipid antibodies (lupus anticoagulant, anticardiolipin and anti-β2 glycoprotein I) were recorded. Also, brain-reactive antibodies, such as antineuronal antibodies, anti-Ribosomal P, and anti-DWEYS were included in the immunologic data.19
Furthermore, therapeutic information was acquired, including whether the patients were under any anticoagulant and antiplatelet agent and the milligrams/day equivalent of their prednisone dosage, hydroxychloroquine, immunosuppressants, and any biologic drug.
The presence of NPSLE was assessed according to the 1999 American College of Radiology nomenclature20 through the confirmation of the attribution of a neurologic event to SLE according to expert opinion.
The control group with 10 patients who were diagnosed with a neuropsychiatric (NP) event derives from the article by Babayan et al:21 6 patients were diagnosed with a major depressive episode, 1 with anorexia nervosa, 1 with alcohol use disorder, 1 with bipolar disorder, and 1 with obsessive-compulsive disorder.
Neuroradiologic Data
MR imaging examinations were conducted within 1 month of enrollment. The Vantage Titan 3T scanner (Canon Medical Systems), equipped with a 32-channel head coil, was used for brain MR imaging. Patients with contraindications for MR imaging, such as claustrophobia or the presence of incompatible MR imaging devices, were excluded from the study.
The MR imaging protocol included a structural 3D T1-weighted fast-field echo (3D MPRAGE) sequence for morphometric analysis. The 3D MPRAGE was acquired in the axial plane from the cranial vertex to the occipital foramen, with the following parameters: section thickness = 1 mm; matrix = 1024; FOV = 256 × 256; TE = 2.8 ms; TI = 950 ms; TR = 6.3 ms; flip angle = 9°. Patients with brain MR imaging scans positive for incidental clinically significant pathologic findings, such as brain tumors, were excluded from the study.
For the NP control group, MR imaging was performed on a 3T scanner (Magnetom Verio; Siemens) equipped with a 32-channel head coil. The MP2RAGE sequence was acquired for assessment of brain structures with a voxel resolution of 1 mm (isotropic). Most important, these T1-weighted images differ from MPRAGE T1-weighted images because they are uniform and free of other imaging properties (ie, proton density, T2∗) that can affect morphometric measurements. The total acquisition time for MP2RAGE was 8 minutes 22 seconds.21
Volumetric Analysis
The morphometric analysis was performed on anonymized structural 3D MPRAGE scans using the automated Vol2Brain online tool (https://www.volbrain.net).22 This Internet-based system was used for the automated analysis of volumetric brain data, quantifying the global brain volume (intracranial cavity, GM, WM, CSF) as well as the volume and mean cortical thickness of 135 brain structures.
Statistical Analysis
To compare continuous and categoric variables between groups, respectively, we used the Student t test or Mann-Whitney U test (for normally or non-normally distributed data, respectively) and the Fisher exact test. ANCOVA was used to correct for variables. Correlation analysis, using either the Pearson coefficient or Spearman coefficient (for normally or non-normally distributed data, respectively) was performed on every variable. The most correlated MR imaging features with every clinical and immunologic variable were partially correlated with the neurologic events, accounting for the prednisone dose, SLEDAI, the presence of NPSLE, and age and sex as partial correlations. Moreover, the neurologic events were correlated with MR imaging variables, accounting for prednisone dose, SLEDAI, age, and sex.
Ordinary least squares (OLS) regression analysis was performed on every clinical and immunologic continuous feature, considered as the target variable, using the most correlated MR imaging features as the predictor variables. A P value of <.05 was considered statistically significant. All statistical analyses were conducted on Python, Version 3.9.
RESULTS
Patient Characteristics
Twenty-seven patients with a diagnosis of SLE were included in the study, of which 24 were women and 3 were men. The average age was 43 years, with a mean disease duration of 7.8 (SD, 11.9) years (Online Supplemental Data). On average, their SLEDAI-2K score was 8.29, while The Systemic Lupus Erythematosus International Collaborating Clinic Damage Index (SLICC-DI) and PGA were 0.96 and 1.04, respectively. The 14 patients who had ≥1 NP event (6 mood abnormalities, 4 seizures, 3 cerebrovascular accidents, 2 cases of psychosis, 1 movement disorder, 1 acute confusional state) had, on average, a SLEDAI-2K of 10.5, while those who did not experience an NP event of 5.9. Likewise, the patients with NPSLE had a higher PGA and SLICC-DI, as well as, on average, a higher double stranded DNA titer (Online Supplemental Data). Every patient except one was on a steroid regimen (96%), prednisone, while 8 were taking an anticoagulant (29%); 19, hydroxychloroquine (70%); 23, an immunosuppressive agent (85%); and 9, a biologic drug (33%). Other patient characteristics are in the Online Supplemental Data.
Volumetric Analysis
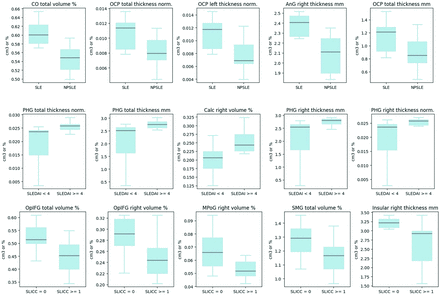
Volumetric differences between various groups were tested. Differences in MR imaging scans of patients with SLE were analyzed by grouping them on the basis of different clinical and immunologic data. Regarding patients with NPSLE and non-NPSLE, many brain regions and their characteristics were found to be different, and the 5 most statistically significant that were lower in the NPSLE group were the following: central operculum of insula total volume percentage (P value = .003), occipital cortex (OCP) total thickness norm (P = .003), OCP left thickness norm (P = .004), angular gyrus (Ang) right thickness in millimeters (P = .004), OCP total thickness in millimeters (P = .005) (Fig 1). When comparing NP events with NPSLE and SLE, with the Wilcoxon test and ANOVA, the brain regions differing the most between the groups were the GRe (gyrus rectus), SGT, and IGt (P < .001).
Wilcoxon rank-sum test results combining multimodal MR brain imaging and machine learning to unravel neurocognitive function in non-NPSLE for volumetric differences between patients with SLE and NPSLE, SLEDAI-2K higher or lower than 4, and SLICC-DI higher or lower than 1. PHG indicates parahippocampal gyrus; OpiFG, opercular part of the inferior frontal gyrus; MPoG, medial precentral gyrus; CO, central operculum of the insula; SMG, supramarginal gyrus.
Regarding the SLEDAI-2K, after the Wilcoxon test, the main differences in MR imaging features between the 2 groups, one with a score of <4 and the other above it, based on studies such as Yee et al,23 were the following: parahippocampal gyrus total thickness greater in higher SLEDAI (P = .006), as well as both its right and left thickness taken separately were greater the higher the SLEDAI (P < .02), and calcarine cortex (Calc) right volume percentage, greater with higher SLEDAI scores. (Fig 2).
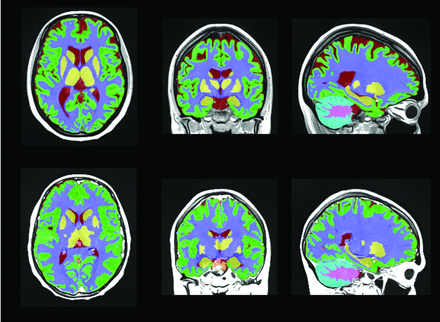
Tissue segmentation of a patient with NPSLE and one with SLE, respectively, first and second row. Each color represents a different brain structure, such as gray and white matter and CSF.
The SLICC-DI analysis, in which the patients were divided on the basis of the presence of organ damage or not (SLICC-DI of at least 1 and 0), revealed how the opercular part of the inferior frontal gyrus total and right volume percentage differed the most between groups (P = .003 and .007), with the damaged group having the smaller volume; another region was the right medial precentral gyrus volume percentage (P = .008), volume in cubic centimeters (P value = .015). (Fig 3).
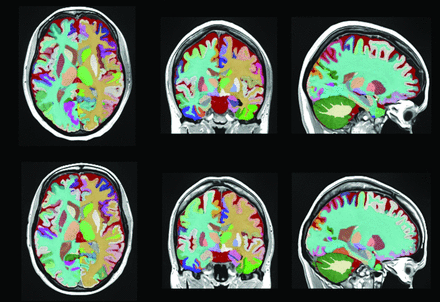
Structure segmentation of a patient with NPSLE and one with SLE, respectively, first and second row. Each color represents a different brain region.
Four patients were diagnosed with antiphospholipid syndrome and were shown to have diminished thickness in the following areas: Anterior insula left thickness in millimeters (P = .001), anterior cingulate gyrus right thickness in millimeters (P = .001), middle temporal gyrus total volume percentage (P value = .001), as well as more abnormal-appearing white matter volume in cubic centimeters (P = .006).
Correlation Analysis
Regarding the partial correlation analysis (Fig 2), the most correlated MR imaging features with NPSLE were the following: subcallosal area (SCA) thickness asymmetry (r = 0.45, P = .003), amygdala right volume percentage (r = 0.43, P = .042), temporal thickness asymmetry (r = 0.47, P = .025), and temporal pole (TMP) thickness asymmetry (r = 0.52, P = .011). Both SCA and TMP thickness asymmetry were positively correlated with the presence of a CVA event after partial correlation (r = 0.71, P < .001 and r = 0.69, P < .001, respectively) (Online Supplemental Data).
On the other hand, the partial correlation analysis of the neurocognitive events revealed a strong positive partial correlation (correlation: 0.662, P < .001) between depression and medial precentral gyrus volume asymmetry, with a negative one for the volume percentage of the cerebellar vermal lobules VIII–X (correlation: −0.5784, P = .004). Similarly, a strong positive partial correlation between seizures and angular gyrus thickness asymmetry (correlation: 0.671, P < .001) was found, as well as a negative one with medial orbital gyrus left thickness norm (correlation: −0.548, P = .006). Our analysis demonstrated a strong negative partial correlation between psychosis and insular right volume percentage (correlation: −0.688, P < .001), in addition to a positive correlation with pallidum total volume in cubic centimeters (correlation: 0.644, P < .001). Furthermore, the correlation with cerebrovascular accidents yielded significant results, such as its relationship with SCA thickness asymmetry (correlation: 0.710, P < .001) and gyrus rectus right thickness in millimeters (correlation: −0.693, P < .001).
OLS Regression
The OLS regression analysis conducted on the continuous variables as the target ones, yielded an R2 of 0.725 and an adjusted R2 of 0.553 for the SLEDAI score, and a R2 of 0.715 and an adjusted R2 of 0.537 for the SLICC-DI score. For SLEDAI, the overall significance of the model, as measured by the F-statistic (F = 4.226, P = .005; F = 4.012, P = .006), indicates that at least one of the predictor variables has a statistically significant effect on SLEDAI-2k and SLICC-DI, respectively.
DISCUSSION
The purpose of this study was to explore the relationship between laboratory values, specifically antibodies, and brain MR imaging findings in patients with SLE. Additionally, it examined the incidence of NP events in connection with brain MR imaging results.
Among our patient cohort, the most prevalent NP manifestations included mood abnormalities, particularly depression, in addition to seizures, psychosis, and cerebrovascular disease. The prevalence of NP events in our population aligns with a meta-analysis by Unterman et al,24 in which the most common neurologic syndromes encompassed headache, mood abnormalities (especially depression), cognitive dysfunction, seizures, and cerebrovascular disease.
Few studies have explored brain MR imaging lesion loads in patients with NPSLE, especially in contrast to controls or patients without NPSLE. Roldan et al25 conducted a retrospective study involving 76 patients with SLE and 26 controls, revealing significantly higher whole-brain and hemisphere lesion loads in patients (all, P < .020). Moreover, a robust association emerged between neurocognitive z scores across all categories and whole-brain and hemisphere lesion loads. Notably, these associations became more pronounced when factoring in glucocorticoid medication and the SLEDAI.
When investigating GM atrophy and its correlation with NPSLE and SLE, an article by Cagnoli et al,26 in which 20 patients with NPSLE, 18 with SLE, and 18 controls, were included, highlighted how GM atrophy was seen in both groups in the temporal and parietal lobes and was most pronounced in the posterior thalamus bilaterally (P < .05); moreover, both groups showed a significant increase in regional GM volume in the posterior parahippocampal gyrus.
A retrospective study by Sârbu et al27 exploring 108 patients with NPSLE elucidated the relationship between WM alterations and NPSLE cognitive and immunologic features. In 59.3% of cases, brain abnormalities were detected. Cerebrovascular syndrome and microbleeds correlated with large-vessel disease, cognitive impairment linked to white matter hyperintensities (WMH), and myelopathy associated with inflammatory-like lesions. Low C4 and CH50 levels were related to inflammatory-like lesions, while lupus anticoagulant levels were related to WMH, microbleeds, and atrophy (P < .010).
In our investigation, the comparison between patients with NPSLE and those without NPSLE revealed the OCP, particularly its total thickness and the left-hemispheric portion, as a potential biomarker distinguishing these 2 groups. Specifically, the total thickness of the OCP and the angular gyrus, along with the total volume percentage of the central operculum of the insula, appeared smaller in the NPSLE group than in the non-NPSLE group. Notably, a statistically significant positive connection emerged between the NPSLE diagnosis and SCA thickness asymmetry, indicating that individuals with NPSLE exhibit greater asymmetry. After adjusting for confounders, every other MR imaging feature retained statistical significance, reinforcing the relationship. Subsequently, these same regions were scrutinized concerning NP events. Both the TMP and SCA thickness asymmetry were associated with cerebrovascular events (P < .050).
The volume of cerebellar vermal lobules VIII–X differed significantly. These findings corroborate those of Mártensson et al,28 who observed lower cerebellar GM density in patients with SLE, especially within cerebellar vermal lobules VIII and VII.
To explore this issue further, we assessed the correlation and partial correlation of cerebellar vermal lobules VIII–X with and in the presence of specific NP events. While the correlation was significant (r = −0.55, P < .001), the adjusted P value slightly exceeded significance (r = −0.40, P = .057). Concerning NP events, the partial correlation with a depressive disorder was notable (r = −0.44, P < .001). These findings align with previous research highlighting the weak correlation between SLE and delayed psychomotor speed,29 a common feature of major depressive disorder, as proved by several studies29,30 including a systematic review by Bennabi et al,31 who pointed out how it might be correlated with the disease severity.
Anti-double stranded DNA antibodies, a pertinent marker in SLE,32,33 were associated with depressive episodes and notably reduced insular, hippocampal, and brainstem volumes. The activation of the complement system, reflected in reduced C3 levels, correlated with diminished precuneus left and total thickness. Intriguingly, Calc right and total volume showed inverse correlations with C3 levels and a direct association with seizures (P < .05).
The impact of antiphospholipid syndrome (APS) on the CNS has been well-documented.34,35 Kaichi et al35 demonstrated a higher prevalence of abnormal MR imaging findings in patients with APS and SLE, including large territorial infarcts, lacunar infarcts, and those with cortical localization (P = .010), compared with the CNS in patients with SLE without APS. Our findings confirm a moderate association between APS and the presence of WM abnormalities (r = 0.47, P < .001). WMH have been shown to correlate with neurocognitive disfunction in patients with NPSLE, as shown by Monahan et al,36 because they reported that lower total brain volume and GM volume are associated with lower cognitive functioning in all domains (P < .01). In our study, we observed significant positive correlations between CVA events and white matter abnormalities (r = 0.44, P = .041), as well as depressive episodes, suggesting that their pathogenesis could be vascular or inflammatory, as discussed by Huang et al,37 along with a recent review by Leal Reato et al,38 highlighting its multifaceted pathophysiology. Notably, we also discovered relationships between these events and GM features, including SCA thickness asymmetry, TMP thickness asymmetry, and right frontal operculum of the insula thickness.
Regarding the other NP events, one of the few studies that investigated their association with WMH or GM hyperintensities has been conducted by Arinuma et al,39 in which 53 patients with NPSLE underwent brain MR imaging, and the frequency of brain lesions, present in 25 of them, for different NP events was assessed. Most of the events were associated with WMH and GM atrophy, with marked severity in almost one-half of them. Events such as psychosis and mood disorders were rarely associated with MR imaging abnormalities in this article, while we found that depressive episodes were positively correlated with features like medial precentral gyrus volume asymmetry (P < .001), and negative correlations were observed with features related to the OCP thickness (P = .002); instead, insular (P < .001) and frontal volumes (P = .001) were negatively correlated with psychotic events, and superior temporal gyrus (P = .004) and cuneus (P = .010) thickness asymmetry was directly correlated with movement disorders, contrary to FO right volume (P = .014), which was lower in such patients.
Furthermore, to better assess the impact of NP events on their own on brain volumes, we tested whether the NP group significantly differed from the NPSLE and SLE groups. Indeed, our volumetric analysis revealed that different brain regions differed between the NP and the other groups, such as GRe, SGT and IGT (P < .001), instead of those that stood out when comparing NPSLE and SLE, suggesting that there is a difference between patients with NPSLE and those with NP disorders.
To conclude our analysis, we used an OLS regression model to further assess MR imaging and clinical variable association (Online Supplemental Data). Notably, Calc right volume percentage has a positive coefficient of 63.0838 with a significant P value of .035, suggesting that an increase in Calc right volume percentage is associated with higher SLEDAI-2k scores. Inferior occipital gyrus volume asymmetry and SCA left thickness in millimeters both have coefficients with P values slightly above the typical significance threshold of .05 (P = .061 and .151, respectively). Medial precentral gyrus right volume percentage (P = .01) was negatively associated (−68.4273) with SLICC-DI, indicating a decrease in its volume for higher scores.
This last step adds to the increasing literature on machine learning applications in NPSLE, with MR imaging data, as shown by recent articles such as the one by Tay et al,40 in which a multimodal model, constructed using machine learning and incorporating microstructural, perfusion, and permeability parameters, effectively predicted the neurocognitive performance of individuals with SLE.
A limitation of this study is the difference in imaging acquisition between the 2 data sets, because it can affect morphometric measurements.
CONCLUSIONS
These findings confirm the interplay between brain structural attributes and NP events, suggesting that quantitative MR imaging–based biomarkers could be helpful in stratifying this condition. However, further research is warranted to validate and extend these preliminary observations.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 9, 2023.
- Accepted after revision January 18, 2024.
- © 2024 by American Journal of Neuroradiology