Abstract
BACKGROUND AND PURPOSE: Collaterals are important in large vessel occlusions (LVO), but the role of carotid artery disease (CAD) in this context remains unclear. This study aimed to investigate the impact of CAD on intracranial collateralization and infarct growth after thrombectomy in LVO.
MATERIALS AND METHODS: All patients who underwent thrombectomy due to M1 segment occlusion from 01/2015 to 12/2021 were retrospectively included. Internal carotid artery stenosis according to NASCET was assessed on the affected and nonaffected sides. Collaterals were assessed according to the Tan score. Infarct growth was quantified by comparing ASPECTS on follow-up imaging with baseline ASPECTS.
RESULTS: In total, 709 patients were included, 118 (16.6%) of whom presented with CAD (defined as severe stenosis ≥70% or occlusion ipsilaterally), with 42 cases (5.9%) being contralateral. Good collateralization (Tan 3) was present in 56.5% of the patients with ipsilateral CAD and 69.1% of the patients with contralateral CAD. The ipsilateral stenosis grade was an independent predictor of good collateral supply (adjusted OR: 1.01; NASCET point, 95% CI: 1.00–1.01; P = .009), whereas the contralateral stenosis grade was not (P = .34). Patients with ipsilateral stenosis of ≥70% showed less infarct growth (median ASPECTS decay: 1; IQR: 0–2) compared with patients with 0%–69% stenosis (median: 2; IQR: 1–3) (P = .005). However, baseline ASPECTS was significantly lower in patients with stenosis of 70%–100% (P < .001). The results of a multivariate analysis revealed that increasing ipsilateral stenosis grade (adjusted OR: 1.0; 95% CI: 0.99–1.00; P = .004) and good collateralization (adjusted OR: 0.5; 95% CI: 0.4–0.62; P < .001) were associated with less infarct growth.
CONCLUSIONS: CAD of the ipsilateral ICA is an independent predictor of good collateral supply. Patients with CAD tend to have larger baseline infarct size but less infarct growth.
ABBREVIATIONS:
- CAD
- carotid artery disease
- LVO
- large vessel occlusion
In acute ischemic stroke due to large vessel occlusion (LVO), collateral pathways play a decisive role in preventing extensive infarction.1,2 Primary collaterals (circle of Willis) and/or secondary collaterals (eg, ophthalmic or leptomeningeal arteries) are activated to ensure that the cerebral oxygen demands are met.3⇓⇓-6 However, the extent of collateralization is highly variable.
Numerous factors, such as age, diabetes, small vessel disease, arterial hypertension, and metabolic syndrome have been identified to impact collateral status.7⇓⇓-10 However, there are heterogeneous study results regarding the correlation of ICA stenosis and collateralization. Several of these studies indicate that pre-existing carotid artery disease (CAD) might promote collateral formation in patients with subsequent ischemic stroke.11⇓⇓⇓⇓-16 The physiologic rationale for this is that chronic hypoxia stimulates blood vessel formation.17⇓⇓-20
However, the inclusion criteria were heterogeneous. Some studies included terminal ICA occlusions, potentially compromising collateral recruitment through the circle of Willis. In addition, some included M2 segment occlusions, which might also lead to a bias.11⇓⇓⇓-15
In addition, the pathophysiological relationship between CAD, collaterals, and infarct growth remains unclear, as none of these studies examined infarct progression. Furthermore, they did not evaluate the contralateral ICA in terms of stenosis degree, neglecting its possible impact on collateralization.11⇓⇓⇓-15
Understanding the relationship of CAD, collateralization, and infarct growth is of not only pathophysiological interest but also clinical interest, as it is still not clear whether patients with stroke with tandem occlusion and large ischemic core benefit from endovascular treatment.
Therefore, the aim of this study is to investigate the impact of ipsilateral and contralateral carotid artery stenosis on intracranial collateralization, initial infarct size, and infarct growth after thrombectomy. We hypothesize that CAD reduces infarct growth in LVO by promoting better intracranial collateralization.
MATERIALS AND METHODS
Study Design
This is a retrospective, observational single-center study. Approval was obtained from the Ethics Committee of the Medical Faculty of Heidelberg University, Heidelberg, Germany. The need for informed consent was waived by the local ethics committee in view of the retrospective nature of the study, and all of the procedures being performed were part of routine care.
All patients undergoing endovascular stroke treatment are prospectively recorded in our institutional thrombectomy registry. This registry was screened for all patients from January 2015 to December 2021. Clinical information was retrospectively collected from medical reports.
The inclusion criteria were: 1) availability of nonenhanced CT and CTA before thrombectomy, and 2) an acute occlusion of the M1 segment of the middle cerebral artery on preinterventional CTA. The M1 segment was defined as the horizontal segment of the middle cerebral artery from the internal carotid bifurcation to the distal genu of the middle cerebral artery, as described by Fischer in his anatomic studies in 1938.21,22
Patients were excluded if a dissection of the carotid artery was present or if the stenosis grade, infarct size, and collateralization were not assessable on CT or CTA. In addition, patients with concomitant occlusion of the intracranial carotid terminus (carotid T or L occlusions) were excluded due to the potential impact on the circle of Willis and infarct progression.23,24 Furthermore, assessing carotid stenosis on CTA can be difficult or even impossible in patients with carotid terminus occlusion due to the common occurrence of pseudoocclusion. Patients with ≥M2 occlusions were also excluded because the extent of leptomeningeal collateral recruitment may have been affected and could not be accurately assessed.
All patients were treated according to national guidelines and in-house standards. This article was written according to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
Image Acquisition
Nonenhanced CT and CTA images were acquired at various CT machines within our regional stroke network. Although the technical CT parameters may vary, the image quality was similar, overall. Follow-up imaging (CT or MR imaging) was usually done 1 day after the thrombectomy.
Image Evaluation
The degree of stenosis was assessed on CTA for the ipsilateral and contralateral ICA, according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET).25 NASCET grading was originally based on conventional angiography but has been reported to show comparable results to those obtained via CTA.26⇓⇓-29 In the present study, stenosis grading was evaluated on CTA, analogously to the work of Randoux et al28; briefly, the degree of stenosis was determined on the plane that projected the most severe stenosis. Carotid artery disease was defined as a severe stenosis (≥70%) or occlusion of the proximal ICA.
Collaterals were assessed according to the Tan collateral score on the same single-phase CT angiography images. Briefly, the collateral status of the affected hemisphere was compared with that of the nonaffected hemisphere. The score reaches from 0 to 3, with 0 indicating no collaterals, 1 indicating the collateralization of 1%–50% of the occluded MCA territory, 2 indicating the collateralization 51%–99% of the occluded MCA territory, and 3 indicating 100% collateralization.30 Good collateralization was defined as a Tan score of 3, as Tan score 2 covers too broad of a spectrum; for example, 52% is considered equivalent to 95% (Fig 1).
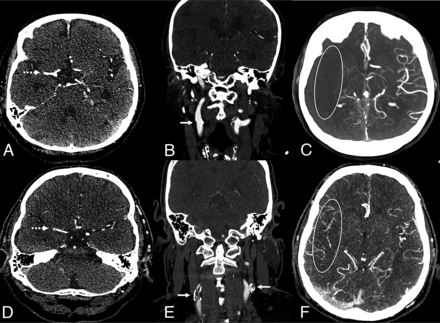
Two exemplary cases of good and poor collaterals in patients with acute right side M1 occlusion of the middle cerebral artery (A and D, dotted arrow). Patient 1 shows no ipsilateral or contralateral stenosis of the extracranial ICA (B, straight arrow) and poor intracranial collaterals, Tan score 0 (C). Patient 2 demonstrates an ipsilateral and contralateral carotid artery disease marked with an arrow (E) with good intracranial collateralization, Tan score 3 (F).
The assessments of the NASCET and Tan scores were conducted by a neuroradiologist and double checked in multiple complicated cases by a second neuroradiologist.
The Alberta Stroke Program Early CT Score (ASPECTS) was initially prospectively assessed on preinterventional and follow-up imaging, and the ratings were checked again for this study. Infarct growth was defined as a decrease of more than 1 ASPECTS point on follow-up imaging compared with the baseline ASPECTS.
Statistics
The statistical analysis was performed using R version 4.2.3 and RStudio. After calculating the descriptive statistics of the data, the correlation between the stenosis grade and collateral grade was analyzed using the Spearman rank correlation. ASPECTS and infarct growth were compared between patients with and without CAD via the Mann-Whitney U test for continuous data. Univariate regression analyses were performed to identify predictors of good collateralization (Tan score 3) and infarct growth (ASPECTS decay of more than 1 point). Variables with a P value of <.05 were used in a multivariate analysis. A P value of <.05 was considered to be indicative of a statistically significant result.
RESULTS
Baseline Characteristics
Between 2015 and 2021, 876 patients with an occlusion of the M1 segment of the MCA were treated and recorded in our institutional registry. Fifty-five patients were excluded because preinterventional CTA was not available. Four patients were excluded due to a dissection of the internal carotid artery on preinterventional imaging. Furthermore, an additional 108 patients had to be excluded due to motion or dental implant artifacts or due to insufficient contrast enhancement on CTA. Thus, a total of 709 patients were included in this study. Retrospectively collected demographic data and clinical information from medical reports are presented in Table 1.
Demographics
Association of Stenosis Degree and Collaterals
Of the eligible 709 patients, 421 (59.4%) showed no carotid stenosis (NASCET 0%) ipsilaterally, and 456 patients (65.3%) presented no carotid stenosis contralaterally. There were 118 patients (16.6%) who had ipsilateral CAD, ie, either a severe stenosis (≥70%) or occlusion ipsilaterally. In 42 cases (5.9%), the contralateral ICA had a severe stenosis or occlusion.
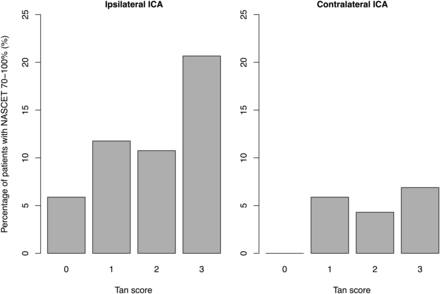
Average Tan score points were slightly increasing with each stenosis grade (Table 2). For patients with ipsilateral CAD, 56.5% presented with good collaterals (Tan score 3), whereas this value was 69.1% for patients with contralateral CAD (Fig 2). In cases with bilateral CAD (n = 22), 66.7% presented with good collaterals.
Tan collateral score distribution in patients with ipsilateral and contralateral carotid artery disease.
Distribution of stenosis grading (NASCET)
There was no rank correlation between the Tan collateral score and ipsilateral (rho = 0.11) or contralateral carotid stenosis degree (rho = 0.09). Still, the ipsilateral stenosis degree was an independent predictor of good collateral supply in a multivariate analysis (adjusted OR: 1.01 per NASCET point; 95% CI: 1.0–1.01; P = .009), whereas the contralateral stenosis degree was not (P = .34) (Table 3). Ipsilateral CAD was associated with better collateralization (unadjusted OR: 2.16; 95% CI: 1.40–3.40; P < .001).
Univariate and multivariate analysis regarding the prediction of good collaterals (Tan 3)
Infarct Growth
Patients with CAD of the ipsilateral ICA showed less infarct growth (median: 1; IQR: 0–2) compared with patients without CAD (median: 2; IQR: 1–3) (P = .005). However, baseline ASPECTS was lower in patients with CAD compared with patients without CAD (P < .001). Follow-up ASPECTS was similar in both groups (P = .337). Contralateral CAD did not show an association with infarct size or growth (Table 4). A multivariate analysis revealed that an increasing ipsilateral carotid stenosis degree (adjusted OR: 1.0; 95% CI: 0.99–1.0; P = .004) and good collateralization (adjusted OR: 0.5; 95% CI: 0.4–0.62, P < .001) were associated with less infarct growth (Table 5).
Distribution of ASPECTS and infarct growth
Univariate and multivariate analysis regarding the prediction of infarct growth
DISCUSSION
Because collaterals are considered an important prognostic factor in large vessel occlusion, factors influencing the collateral status are of high interest.1,2 There are heterogeneous data on the correlation of CAD with the intracranial collateral supply.11⇓⇓⇓⇓-16 Until now, the pathophysiological role of CAD in collateralization was unclear. According to our results, ipsilateral CAD is associated with good collateralization. However, there is no rank correlation of the stenosis degree with the collateral grading, ie, patients with stroke with a concomitant 20% ICA stenosis do not necessarily have better collaterals compared with patients with a 10% stenosis.
Furthermore, we could demonstrate a positive association of ipsilateral CAD with infarct growth independent of collateralization. This means that patients with CAD and poor collaterals show less infarct progression than do patients without CAD.
Hence, our data, with the largest cohort (n = 709), confirms the study results of Rebello et al14 (n = 122), Pienimäki et al11 (n = 247), Hassler et al12 (n = 281), and Guglielmi et al13 (n = 666), all of whom reported an association between good collateral status and carotid stenosis in their studies.
However, the study designs including patient inclusion criteria have noteworthy differences and potential bias. All 4 studies supporting our results included terminal ICA occlusions, consequently impacting the circle of Willis flow by obstructing a potential blood pathway from the posterior communicating artery to the A1 segment of the anterior cerebral artery or vice versa. Depending on anatomic variation of the circle of Willis, this could have a relevant impact.23 In the cases of Pienimäki et al11 and Guglielmi et al13, it is also unclear how the stenosis degree on these particular patient groups was assessed on CTA, as the pseudo-occlusion of the ICA are frequently observed in patients with terminal ICA occlusions. Rebello et al14 solved this issue by using DSA, and Hassler et al12 also mention that they validated their results with DSA.
Furthermore, Hassler et al12 and Guglielmi et al13 included M2 occlusions, while also using the Tan score for collateral status evaluation. This potentially increased the number of patients with good collateral supply, as they considered Tan 2 as good collateral status. Rebello et al14 also included M2 occlusions while using the Souza score, which is a modified version of the Tan score. Nevertheless, there was also a potential increase of patients with better collaterals, as dichotomized between a Souza score of 0–1 (absent/poor collaterals) and a Souza score of ≥2 (moderate/good collaterals).
We tried to homogenize our cohort by solely including intracranial M1 occlusions and only considering Tan 3 as good collaterals, similarly to Pienimäki et al11, who considered Souza 3 and 4 as very good collaterals and statistically conjoined them due to a small number of patients having a Souza score of 4.11⇓⇓-14,31
Contrary results were reported by Dankbaar et al15 and Sobczyk et al.16 Dankbaar et al15 analyzed 188 patients with M1 occlusion from the prospective Dutch Acute Stroke Trial and found no correlation between stenosis degree and collateralization. However, only 18 patients had a stenosis of ≥70%. As pointed out by Pienimäki et al11, this is a relatively small number of patients compared with their study of 51 patients (approximately 20%) with ≥75% stenosis. In our cohort, 118 patients (approximately 17%) presented with carotid stenosis of ≥70%. Therefore, there is a higher likelihood of bias in the data of Danbkaar et al.11,15 This correlation is also supported by the results of Guglielmi et al13, who report a slightly better collateralization in patients with severe carotid stenosis (>70%) compared with moderate stenosis (51%–70%).
However, Sobczyk et al16 had a completely different study design and did not investigate patients with LVO. They included 58 patients with different stenosis degrees from their database and primarily investigated cerebrovascular reactivity. They concluded that collateralization varies greatly and cannot be predicted solely based on the degree of carotid stenosis.
As pointed out initially, we could demonstrate a positive association of ipsilateral CAD with infarct growth independent of collateralization. Patients with CAD and poor collaterals still had a lower rate of infarct progression than did patients without CAD. To our knowledge, this is the first report of a correlation between CAD and infarct growth.
Interestingly, while patients with ipsilateral CAD had a greater baseline infarct size, infarct growth was smaller compared with patients without ipsilateral CAD. In the end, follow-up ASPECTS was similar in patients with and without CAD in our cohort. To our knowledge, this particular finding has not been reported previously, and it stands in contrast to the study results by Deng et al32, who, in their retrospective study of 158 patients who underwent thrombectomy, demonstrated that greater baseline ASPECTS was associated with less infarct growth. However, they did not differentiate between patients with CAD.
The pathophysiological correlation between CAD and collateralization/infarct growth is not yet fully understood. One possible explanation for the lower baseline ASPECTS could be that patients with initially activated collaterals due to CAD have impaired reserve capacities.5,16 However, in theory, the chronic hypoxic state caused by CAD may have preconditioned neurons to hypoxia, which ultimately prolonged their resilience in the event of an acute intracranial large vessel occlusion.33 This hypothesis is supported by an animal experiment by Choi et al34, who experimented on rats with either bilateral common carotid artery ligation or sham operation that were exposed to MCA occlusion and reperfusion. Conclusively, rats with bilateral common carotid artery ligation had smaller infarcts, less DNA-damaged cells, increased cellular defense mechanisms, and evidence of extracellular matrix remodeling. Another possible explanation for the association of CAD and collateralization is that a chronic cerebral hypoxic state stimulates arteriogenesis and angiogenesis, as suggested by Busch et al18 and Kahn et al17, based on animal experimental studies. Similar results were reported by Ohtaki et al19 and Hai et al20, who observed angiogenesis in chronic cerebral hypoperfusion due to an upregulation of vascular endothelial growth factor in rat models.
Therefore, in view of clinical practice, our retrospective data suggest that patients with large ischemic core and tandem lesions should not be excluded from endovascular treatments, as infarct growth seems to be less present in patients with CAD. This is notable, considering that patients with tandem occlusions are typically more technically challenging and take longer to recanalize than do those with isolated MCA occlusions.35
This finding is of particular interest, as the 6 randomized controlled trials on thrombectomy in patients with large ischemic core were in favor of thrombectomy. Only 3 of the 6 large core trials included cervical tandem occlusions.36⇓⇓⇓⇓-41 Further studies and post hoc analyses are necessary to understand the relevance of tandem lesions.
In contrast to other studies, we also analyzed the contralateral ICA for the presence of CAD and its relationship with collateral status and infarct growth. According to our results, contralateral CAD is associated with infarct growth, but it is not an independent predictor when taken together with ipsilateral CAD. Regarding this topic, Maus et al42 retrospectively analyzed 197 patients with tandem occlusion who were undergoing endovascular therapy. Even though they did not explicitly investigate infarct progression, their results suggest an adverse effect of contralateral stenosis on clinical outcome. They reported that the presence of a contralateral carotid stenosis of >50% was associated with a worse clinical outcome.42 However, further studies are necessary.
The strengths of our study are its large cohort size with 709 patients and its assessment of not only ipsilateral but also contralateral ICA regarding CAD. In addition, to our knowledge, this is the first report of a correlation between CAD and infarct growth. We potentially avoided multiple pitfalls by only focusing on intracranial M1 occlusions. By excluding terminal ICA occlusions, we prevented the potential impairment of collateral recruitment through the circle of Willis and avoided issues with stenosis assessment with pseudo-occlusion. By excluding M2 and distal occlusions, we made the assessment of collateral more distinct.
However, this study has some limitations. The retrospective design of our study may have led to selection bias. Our study was limited to patients with M1 segment occlusions and therefore may not be applied to other occlusion locations. Another bias may have resulted from the use of conventional (single phase) CTA for the collateral assessment, as it is static and only displays a single momentum in the arterial phase. However, multiphase or dynamic CTA was not available for every patient. Excluding patients without multiphase or dynamic CTA would have led to a selection bias. Moreover, nonenhanced CT and CTA images were acquired using various CT machines within our regional stroke network. Even though the image quality was similar overall, it may have resulted in a bias. In addition, CT or MR imaging was used for follow-up imaging, which may have led to a bias in ASPECTS interpretation.43 Moreover, we did not distinguish between different onset times to imaging. As such, this may have an impact, especially on the initial ASPECTS, as reported by Potreck et al.44 Similarly, the exact time of follow-up imaging was not recorded in our institutional thrombectomy registry. It is usually done 1 day after thrombectomy, but this time interval might have an impact on the assessment of the postinterventional infarct size.
CONCLUSIONS
CAD of the ipsilateral ICA, but not the contralateral ICA, is an independent predictor of good collateral supply. Patients with ipsilateral CAD tend to have larger baseline infarct sizes but less infarct growth. Ultimately, follow-up infarct size is similar overall in patients with and without CAD. Our results suggest that CAD has an influence on infarct growth. Hence, patients with CAD should not be excluded from endovascular treatments, despite large infarct sizes on admission.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received October 9, 2023.
- Accepted after revision January 11, 2024.
- © 2024 by American Journal of Neuroradiology