Abstract
BACKGROUND AND PURPOSE: A global decrease in brain perfusion has recently been reported during exposure to a ground-based spaceflight analog. Considering that CSF and glymphatic flow are hypothesized to be propelled by arterial pulsations, it is unknown whether a change in perfusion would impact these CSF compartments. The aim of the current study was to evaluate the relationship among changes in cerebral perfusion, ventricular volume, and perivascular space volume before, during, and after a spaceflight analog.
MATERIALS AND METHODS: Eleven healthy participants underwent 30 days of bed rest at 6° head-down tilt with 0.5% atmospheric CO2 as a spaceflight analog. For each participant, 6 MR imaging brain scans, including perfusion and anatomic-weighted T1 sequences, were obtained before, during, and after the analog period. Global perfusion, ventricular volume, and perivascular space volume time courses were constructed and evaluated with repeated measures ANOVAs.
RESULTS: Global perfusion followed a divergent time trajectory from ventricular and perivascular space volume, with perfusion decreasing during the analog, whereas ventricular and perivascular space volume increased (P < .001). These patterns subsequently reversed during the 2-week recovery period.
CONCLUSIONS: The patterns of change in brain physiology observed in healthy participants suggest a relationship between cerebral perfusion and CSF homeostasis. Further study is warranted to determine whether a causal relationship exists and whether similar neurophysiologic responses occur during spaceflight.
ABBREVIATIONS:
- BDC
- baseline data collection
- HDT
- head-down tilt
- ICP
- intracranial pressure
- PVS
- perivascular spaces
- R
- recovery
- VaPER
- Visual Impairment Intracranial Pressure and Psychological :envihab Research
Spaceflight is associated with various environmental stressors including the absence of normal gravity, chronic exposure to altered atmospheric compositions, and reduced sensory input. Study of the body's physiologic responses to these challenges will advance future space exploration and may provide insight into normal function on Earth. Following spaceflight, brain MR imaging has revealed structural changes such as upward shift of the brain,1,2 narrowing of the vertex CSF spaces,3 increased ventricular volume,3⇓⇓-6 enlargement of perivascular spaces (PVS),7,8, and redistribution of free water.9
Modeling the spaceflight environment on Earth is challenging. Space agencies have commonly used a 6° head-down tilt (HDT) bed rest as an earth-based analog to study the effects of microgravity on the body. By reversing the gravitational vector in the z-direction toward the head compared with toward the feet in the normal daily upright position, HDT simulates certain physiologic changes of spaceflight including the following: unloading of the lower body, altered sensory input, and cephalad fluid shifts.10,11 However, many have noted shortcomings of HDT as a direct spaceflight analog.10,12 In 2017, the National Aeronautics and Space Administration (NASA) and the German Space Agency performed a 30-day bed rest study, known as the Visual Impairment Intracranial Pressure and Psychological :envihab Research (VaPER) study, which improved on past bed rest protocols by exposing participants to elevated CO2 levels to mimic the International Space Station conditions and enforcing a “strict” HDT position throughout the intervention.13 In the multi-investigator VaPER bed rest study, several researchers have documented alterations in brain function and behavioral performance.14⇓⇓-17 Of relevance to the current study, Roberts et al16 documented a mean decrease in global relative brain perfusion during the bed rest period.
However, the relationship between reduced brain perfusion and other physiologic variables such as ventricular volume and PVS has not been examined in participants in VaPER. Previous work has shown that both parameters are sensitive to spaceflight3,5,7,8 and additionally, that PVS has evidence of links to decreased cerebral perfusion.18,19 Therefore, the purpose of this study was to expand on previous findings by examining concurrent changes in perfusion, ventricular volume, and PVS during HDT and recovery. We hypothesized that decreased perfusion would be accompanied by an increase in ventricular and PVS volumes, like that seen in astronauts. Understanding changes in cerebral physiology that occur in response to the unique physiologic stressor of altered gravity is vital for ensuring optimal performance and safety for continued space exploration, while also providing insight to better understand fundamental cerebral structure and function in patients on Earth.
MATERIALS AND METHODS
Participants
Eleven healthy participants (6 men, 5 women; median age, 33 years; median absolute deviation = 6) participated in the VaPER study conducted in Cologne, Germany, at the :envihab facility of the German Aerospace Center (Deutsches Zentrum für Luft-und Raumfahrt). Participants provided written informed consent, and the study was approved by the ethics commission of the local medical association (Ärztekammer Nordrhein) and institutional review boards at NASA and the Medical University of South Carolina. All participants underwent routine health screening as previously reported, and all were nonsmokers for at least 6 months before the start of the study.14
Study Protocol
A detailed protocol of the multi-investigator study has been described previously.13,16,17 Briefly, participants began their stay at the :envihab facility 14 days before the bed rest portion of the study for baseline data collection (BDC), while remaining ambulatory under normal atmospheric conditions. Next, they underwent 30 days of 6° HDT bed rest in a 0.5% CO2 environment (HDT + CO2). Participants maintained the HDT position at all times, including while eating, and were continually monitored via video to ensure compliance. Finally, they recovered (R) in the facility for 14 days postbed rest, returning to normal atmospheric and ambulatory conditions. MR imaging was performed at 6 time points: 13 (BDC-13) and 7 (BDC-7) days before bed rest, on days 7 (HDT7) and 29 (HDT29) during bed rest, and 5 (R + 5) and 12 (R + 12) days after bed rest during recovery. Throughout the study, participants were given standardized meals to maintain body weight and standardized daily water consumption levels based on their weight.20 Participants were not allowed to have caffeinated beverages.20 For scans during the analog period, participants were placed on a foam wedge on the MR imaging table to strictly maintain the HDT position and supplied CO2 at 0.5% via a mask to maintain the same CO2 exposure throughout the MR imaging examinations. Following the precedent of prior reports, BDC-13 was considered an acclimation time point, and BDC-7 was considered the pre-bed rest time point.14,15,17
MR Imaging Protocol
MR imaging was performed at 3T (Biograph mMR, software, Version VE11P; Siemens). The protocol included a 3D T1-weighted gradient-echo pulse sequence for anatomy (192 slices, 0.94 × 0.94 × 0.90 mm, FOV = 270 × 270 mm, TR = 1.9 seconds, TE = 2.49 ms, flip angle = 9°) and pulsed ASL using 3D gradient/spin-echo sequences with background suppression, flow-sensitive alternating inversion recovery (FAIR) labeling, and quantitative imaging of perfusion with a single subtraction with thin-section TI1 periodic saturation (Q2-TIPS) bolus saturation (40 slices, 1.5 × 1.5 × 3 mm voxel resolution, FOV = 192 × 192, TR = 4600 ms, TE = 16.38 ms, flip angle = 180°). Four control-label pairs were acquired with a 700-ms pulse duration and a 1990-ms postlabeling delay. This sequence, which was the only ASL perfusion sequence available on the :envihab MR scanner, did not include calibration imaging needed for CBF quantification. Thus, perfusion-weighted maps were globally scaled with an arbitrary value of M0 = 1000. Therefore, as previously, relative perfusion values are reported.16
Image Processing
Detailed image-processing methods are included with Online Supplemental Data and briefly described here.
PWIs were created from ASL data using the FMRIB Software Library (FSL, Version 6.0.3; (http://www.fmrib.ox.ac.uk/fsl) as previously described.16 Global whole-brain PWI values were extracted from the masked perfusion maps to statistically evaluate the mean perfusion of each subject. Segmentation and calculation of ventricular volumes were performed using FreeSurfer Recon-all (Version 6.0.0; http://surfer.nmr.mgh.harvard.edu) on T1-weighted structural brain images.21 A sum of the lateral and third ventricle volumes was calculated and hereafter is referred to as our measure of ventricle volume. The fourth ventricle was omitted due to previous work showing that its volume was unchanged by spaceflight.5,6 WM perivascular space (WM-PVS) segmentation was performed on the parcellations in native space previously obtained from FreeSurfer via an automated pipeline,22 as in previous studies.22⇓-24 In this study, we focused solely on WM-PVS and not the basal ganglia PVS because the widespread orientation of WM-PVS aligned more closely with our interest in global perfusion.
Statistical Analyses
Age is described with median and median absolute deviation. Z-scores [z = (x – mean) / (SD)] were calculated across time for each subject to account for individual differences and facilitate comparisons between metrics measured on different scales. A repeated measures ANOVA was performed on the subjects' z-scores for each time point for global relative perfusion, ventricular volume, and PVS volume. Partial eta-squared (η2p) effect sizes were reported. We evaluated 5 time points: BDC-7, HDT7, HDT29, R + 5, and R + 12. Pair-wise comparisons were used to evaluate changes in brain metrics for the following a priori comparisons of interest: from baseline to the end of bed rest (BDC-7 versus HDT29), from baseline to recovery (BDC-7 to R + 12), and between brain metrics at these times (BDC-7, HDT29, R + 12). Pair-wise effect sizes were calculated with the Hedges' g and were interpreted as very small (g = 0.01), small (g = 0.20), medium (g = 0.50), large (g = 0.80), very large (g = 1.20), and huge (g = 2.00)25 and were reported with 95% CIs. Because arterial pulsations are thought to propel CSF along the PVS, a Spearman rank correlation was performed between individual changes in perfusion and changes in PVS z-scores from baseline (BDC-7) to the end of bed rest (HDT29). Statistical significance was set at the α < .05 threshold, and analyses were conducted with R statistical and computing software, Version 4.1.2 (http://www.r-project.org/) and SPSS, Version 27 (IBM).
RESULTS
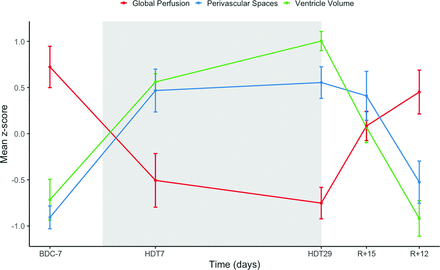
Mean raw perfusion, ventricle volume, and PVS values are provided in Table 1 and participant examples are provided in Figures 1⇓–3. A significant interaction revealed differing trajectories for perfusion, PVS volume, and ventricular volume as time progressed from before HDT + CO2 to recovery, (F(8, 80) = 11.08; P < .001; η2P = 0.53) (Table 2 and Fig 4).
Raw values for brain metrics at each time pointa
Z-score scaled brain metrics at each time pointa
Baseline Differences among Perfusion, Ventricular Volume, and PVS
Relative to the mean level across time for each metric, at baseline, perfusion was greater than ventricular volume (P < .001; g = 1.49; 95% CI, 0.61–2.33) and PVS volume (P < .001; g = 1.57; 95% CI, 0.67–2.43), and PVS volume did not differ from ventricular volume (P = .440; g = 0.23; 95% CI, −0.35–0.81).
Brain Changes during HDT
By the end of the HDT + CO2 period, there was an increase in ventricular volume (P < .001; g = 1.92; 95% CI, 0.90–2.92; Fig 2) and PVS volume (P < .001; g = 2.64; 95% CI, 1.36–3.90; Fig 3), whereas perfusion decreased (P < .001; g = 1.78; 95% CI, 0.81–2.72; Fig 1) compared with baseline. A negative change-change correlation with perfusion and PVS was observed from BDC-7 to HDT29, indicating that a larger increase in PVS volume was associated with a larger decrease in perfusion (rs[9]) = −0.77, P = .008).
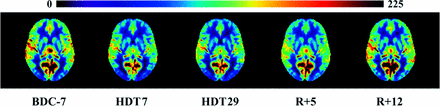
Mean perfusion-weighted images using masks of all participants at each time point throughout the study. Yellow/red indicates greater perfusion on arbitrary scaled units.
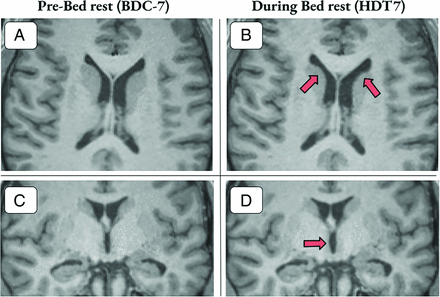
T1 images with examples of ventricular volume enlargement for 2 participants 7 days into bed rest. A and B, Axial section with arrows highlighting areas of gross ventricular enlargement in the lateral ventricles. C and D, Coronal section with arrows highlighting enlargement of the third ventricle. Participants shown experienced a 9.12% and 12.31% increase in ventricular volume, respectively.
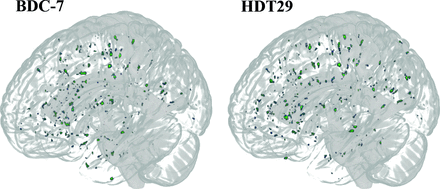
3D mask representation of WM-PVS of a sample participant from pre-bed rest (BDC-7) to the end of bed rest (HDT29). The following participant experienced a 12% increase in PVS volume.
Brain Recovery from HDT
The divergent trajectories of brain metrics continued during recovery as ventricular volume (P < .001; g = 2.67; 95% CI, 1.38–3.95) and PVS volume (P = .012; g = 0.89; 95% CI, 0.19–1.56) decreased from HDT29 to R + 12, whereas global perfusion increased during recovery (P = .006; g = 1.01; 95% CI, 0.28–1.71; Table 2 and Fig 4). At the end of recovery (R + 12), their relative positions shifted with global perfusion significantly greater than both PVS volume (P = .025; g = 0.76; 95% CI, 0.09–1.41) and ventricular volume (P = .004; g = 1.07; 95% CI, 0.32–1.78) with no difference between PVS and ventricular volume (P = .146; g = 0.46; 95% CI, −0.15–1.05).
Global relative perfusion, ventricular volume, and PVS time courses. Gray shaded area represents the duration of the HDT + CO2 intervention. Data points for scanning days include pre- (BDC-7), during (HDT7 & HDT29), and post- (R + 5 & R + 12) HDT + CO2 period and are represented with mean z-scores (standard error of the mean). A divergent trajectory is observed with perfusion decreasing while ventricular and perivascular space volume increase during the HDT + CO2 period.
HDT Differences between Perfusion, Ventricular Volume, and PVS
At HDT29, ventricular volume was greater, relative to its mean across time, than both PVS volume (P = .017; g = 0.83; 95% CI, 0.14–1.49) and perfusion (P < .001; g = 2.05; 95% CI, 0.99–3.09), and PVS volume was greater than perfusion (P < .001; g = 1.47; 95% CI, 0.60–2.30).
Recovery versus Baseline Differences
At the end of recovery, we found no differences compared with the baseline values for ventricular volume (P = .619; g = 0.15; 95% CI, −0.43–0.72), PVS volume (P = .275; g = 0.33; 95% CI, −0.26–0.91), or global perfusion (P = .503; g = 0.20; 95% CI, −0.20 to −0.38). The brain metrics revealed a double dissociation in which ventricular volume and PVS volume increased during HDT+CO2 with a decrease to baseline levels during recovery, whereas global perfusion decreased during the HDT + CO2 period and increased to baseline levels during recovery, suggesting distinct neurophysiological responses to simulated microgravity.
DISCUSSION
The purpose of this study was to investigate changes in cerebral perfusion, ventricular volume, and PVS volume in healthy participants in response to 30 days of HDT + CO2. The main finding was statistically significant changes in brain metrics of opposite directionality, with global perfusion decreasing and ventricular/PVS volumes increasing during HDT + CO2 and subsequent reversal during recovery.
While 2 recent studies found alterations in PVS volumes in astronauts postspaceflight,7,8 this is the first study to report a change in PVS volumes in an HDT microgravity analog setting, and in general, transient PVS dilation and reversal in a cohort of healthy participants. Furthermore, decreased global perfusion in VaPER participants presented by Roberts et al16 is the only previous study examining longitudinal changes in perfusion by MR imaging in a prolonged microgravity analog. A short-duration study found a 17%–20% decrease in CBF after 26.5 hours of 12° HDT measuring carotid and vertebral artery blood flow using a cine phase-contrast MR image, but it did not extrapolate to multiple time points.26 Other HDT studies have investigated measurements of CBF velocity via transcranial Doppler. However, the results of these studies are inconclusive, likely due to limitations of this technique based on the assumption that the cross-sectional area of the interrogated vessel is fixed, which might not be the case, particularly in an environment with altered CO2 levels.16,27 Although a perfusion-PVS link has not been explored in a longitudinal study, the inverse relationship observed in this study is in line with previous results that showed that greater PVS volumes may be associated with decreased CBF in healthy participants and patients with intracranial and extracranial atheroscleroisis.18,19 Moreover, the ventricular volume changes presented here align with previous findings after HDT (a 2%–3% increase in lateral ventricle volume was found after 26.5 hours of HDT)26 and long-duration spaceflight (increases of ∼11% have been found in both astronauts and cosmonauts.) 2,3,5
The mechanisms driving decreased cerebral perfusion and ventricular/PVS enlargement in healthy participants during the 30 days of HDT + CO2 are unknown. However, it is becoming clear that real and simulated microgravity elicit changes in cerebral hemodynamics and CSF dynamics.27,28 In spaceflight and HDT, normal hydrostatic pressure gradients, usually experienced along the head-to-foot axis during upright posture, are reduced, resulting in the observed cephalad fluid shifts.27⇓-29 It has been hypothesized that this upward fluid shift may lead to venous congestion in the head and possibly elevated intracranial pressure (ICP).5,16,28 From a hemodynamic perspective, venous congestion could lead to venous hypertension, elevated ICP, and decreased cerebral perfusion. Indeed, impaired venous outflow has been linked to reduced cerebral perfusion in patients with chronic cerebrospinal venous insufficiency.30⇓-32 Following surgical restoration of normal internal jugular venous flow, patients demonstrated improved brain perfusion and a 9.6% reduction in ventricular size.30 Most interesting, this change in ventricular volume related to venous insufficiency is similar to the ventricular volume change previously reported in astronauts following long-duration spaceflight.1,25 In an astronaut population, a recent article showed postflight increases in superior sagittal, transverse, and sigmoid sinus volumes.33 The authors suggest improper venous drainage due to the absence of gravitational gradients and that this may explain thrombosis and abnormal internal jugular venous flow reported in astronauts.34,35 As an additional contributing factor, cardiac output and stroke volume are likely affected by microgravity. Reviewing the current literature, Bateman and Bateman36 surmised that both are reduced in HDT but increased during spaceflight, which may point to a key physiologic difference between the environments.
Similarly, it is likely CSF homeostasis is altered by reversal of the gravitational gradient. Upward shift of the brain itself has been suggested to impair CSF resorption by compression of the superior sagittal sinus.5,33 In this theory, the ventricular system may then act as a buffer for excess CSF resulting in ventricular enlargement. Similarly, an increase in PVS volume may reflect obstruction or inefficiency in the exchange of CSF and interstitial fluid that occurs in perivascular channels. CSF flow is intrinsically connected to hemodynamics in that transmission of arterial pulsations are a driving force for CSF movement.37 In this view, decreased perfusion could reduce the ability to circulate CSF, with resulting consequences for the perivascular and ventricular compartments. Indeed, cross-sectional studies showing an inverse relationship between cerebral perfusion and PVS size have hypothesized that decreased blood flow leads to increased interstitial fluid around the PVS and subsequent PVS dilation.18 This hypothesis is in line with the negative correlation between perfusion and PVS volume seen in this study. Measurements of ICP could help elucidate the interplay between perfusion and CSF changes, but no direct measurement of ICP during long-duration HDT or microgravity has been performed. However, several studies have reported elevated ICP in the acute stages of HDT or in the transition from upright to supine.38,39 Additionally, several astronauts presented with mildly elevated opening pressures via lumbar puncture (21–28.5 cm H20) months after spaceflight.40
A study limitation was the necessary use of an ASL sequence that did not provide absolute CBF values. The small sample size (n = 11) and large number of relevant study variables reduced our statistical power, though several group-level findings had robust effect sizes. Additionally, various methods for PVS quantification exist, and the automated method used here based on PVS morphologic features has potential limitations, such as partial voluming effects of the enclosed vessel.41 Most important, it is not clear that HDT bed rest represents an accurate terrestrial analog for spaceflight.12
Here, we document an association among patterns of cerebral perfusion, ventricular volume, and PVS volume that occur over a HDT + CO2 intervention and recovery. The findings contribute to our understanding of the relationships among the circulatory, glymphatic, and ventricular systems of the brain, specifically, revealing a possible direct link between PVS volumes and cerebral perfusion demonstrated by a prolonged perturbation of CBF in healthy participants. The results also provide additional evidence of changes in cerebral physiology in response to simulated microgravity. The significance of these changes, if any, should be further explored. For example, alterations in vision and ophthalmologic findings following spaceflight have been reported in astronauts, known as the spaceflight-associated neuro-ocular syndrome and thought to be linked to cephalad fluid shifts and venous congestion.40 Altered cerebral physiology may also play a role in cognitive and performance decrements previously reported in astronauts.3,14,42 Most important, given the increasing number of commercial spaceflight participants who may not be as physically fit as career astronauts and who may have mild forms of cerebrovascular disease, a pronounced decrease in cerebral perfusion without adequate reserve raises the possibility of spaceflight-induced ischemic events. While these data represent one step forward in developing a comprehensive model of the neurophysiologic response to microgravity, ultimately inflight evaluation of cerebral perfusion and ICP in astronauts is greatly needed.
ACKNOWLEDGMENTS
Research reported in this study was facilitated by the Spaceflight Standard Measures Cross-Cutting Project of the Human Research Program of the National Aeronautics and Space Administration. We also thank the entire VaPER study staff.
Footnotes
This study was funded by the National Aeronautics and Space Administration (NASA), grant No. NNX13AJ92G.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received September 16, 2022.
- Accepted after revision June 25, 2023.
- © 2023 by American Journal of Neuroradiology