Abstract
BACKGROUND AND PURPOSE: Arterial spin-labeling is a noninvasive MR imaging technique allowing direct and quantitative measurement of brain perfusion. Arterial spin-labeling is well-established in clinics for investigating the overall cerebral perfusion, but it is still occasionally employed during tasks. The typical contrast for functional MR imaging is blood oxygen level–dependent (BOLD) imaging, whose specificity could be biased in neurologic patients due to altered neurovascular coupling. This work aimed to validate the use of functional ASL as a noninvasive tool for presurgical functional brain mapping. This is achieved by comparing the spatial accuracy of functional ASL with transcranial magnetic stimulation as the criterion standard.
MATERIALS AND METHODS: Twenty-eight healthy participants executed a motor task and received a somatosensory stimulation, while BOLD imaging and arterial spin-labeling were acquired simultaneously. Transcranial magnetic stimulation was subsequently used to define hand somatotopy.
RESULTS: Functional ASL was found more adjacent to transcranial magnetic stimulation than BOLD imaging, with a significant shift along the inferior-to-superior direction. With respect to BOLD imaging, functional ASL was localized significantly more laterally, anteriorly, and inferiorly during motor tasks and pneumatic stimulation.
CONCLUSIONS: Our results confirm the specificity of functional ASL in targeting the regional neuronal excitability. Functional ASL could be considered as a valid supplementary technique to BOLD imaging for presurgical mapping when spatial accuracy is crucial for delineating eloquent cortex.
ABBREVIATIONS:
- ASL
- arterial spin-labeling
- BOLD
- blood oxygen level–dependent
- CMRO2
- cerebral metabolic rate of oxygen
- CoG
- center of gravity
- ESI
- electrical source imaging
- fASL
- functional ASL
- MAX
- global maximum of activation
- TMS
- transcranial magnetic stimulation
fMRI is a noninvasive imaging technique for studying cerebral functions in healthy, clinical populations. Blood oxygen level–dependent (BOLD)1 imaging relies on the complex interplay between neuronal activity and associated blood changes (CBF, CBV, and the cerebral metabolic rate of oxygen [CMRO2]). Therefore, BOLD imaging offers an indirect and nonquantitative measure of neuronal activity. Moreover, BOLD specificity can be biased by the presence of draining veins2 or in the case of pathologic neurovascular coupling, as with brain tumors,3,4 epilepsy,5,6 and cerebrovascular diseases.7
Previous studies compared BOLD localization with other imaging modalities, particularly in sensory and motor experiments. During finger movement, anterior-to-posterior and medial-to-lateral shifts of BOLD imaging with respect to electrical source imaging (ESI) have been found.8 Inuggi et al9 showed that BOLD activation of a tapping thumb localized more posteriorly with respect to transcranial magnetic stimulation (TMS). Similarly, during pneumatic stimulation of the thumb, BOLD imaging localized significantly more laterally and posteriorly than ESI.10
The limitations of BOLD imaging have motivated the development of quantitative MR imaging to characterize the hyperemic (CBF, CBV) and metabolic responses (CMRO2) triggered by neuronal spiking.11,12 Arterial spin-labeling (ASL) is one of the most established MR imaging techniques for absolute quantification of CBF: The perfusion map is obtained by the averaged pair-wise subtraction of brain images acquired alternately in conditions of blood-sensitized (labeled) and static (control) tissue.13,14 Alternatively, from the succession of pair-wise subtractions, the functional time course of perfusion can be extracted,15 implying that ASL can be used as a functional MR imaging technique (functional ASL [fASL]) to quantify the arterial blood delivered to the brain tissue during tasks.
Given the variety of MR imaging sequences,16 the intrinsic low SNR and temporal resolution, and the lack of standardized pipelines,17,18 ASL is slowly emerging in the clinical setting as a functional tool. Nevertheless, ASL has remarkable advantages with respect to BOLD imaging: 1) quantitative measurement relevant for longitudinal19 or pharmacologic studies,20 2) unaffected by venous contamination,21 and 3) insensitive to slow drift.22 Moreover, ASL has a higher intra- and intersubject stability compared with BOLD imaging.23,24
fASL has been investigated in various tasks25⇓-27 and for functional connectivity,28,29 but few publications tackled localization specificity compared with other techniques. Noteworthy, most studies have compared fASL and BOLD imaging using different sessions or relying on the BOLD imaging extracted by the ASL sequence.25,26,30,31 For an appropriate comparison with BOLD imaging in task-based imaging, the selection of the ASL sequence is fundamental.16 Recently, dual-echo pseudocontinuous ASL sequences have been developed, enabling simultaneous acquisition of BOLD imaging and ASL with optimal parameters for both contrasts.27⇓-29,32,33
The translation of fASL into the clinical routine requires a systematic characterization of the advantages and potential disadvantages with respect to the standard BOLD imaging.
In this context, the current work aims to assess the performance of fASL for the delineation of the functional cortex during motor and somatosensory tasks in a cohort of healthy subjects. We used dual-echo pseudocontinuous ASL to obtain, simultaneously, perfusion-based and BOLD images, avoiding intersession variability.34 Also, we attributed our results to the individual hand somatotopy obtained during TMS and linked the information driven by the 2 MR imaging modalities with the motor neuron excitability. In line with previous findings, we hypothesized a significant shift in the localization between BOLD imaging and fASL. Moreover, given the quantitative nature of fASL, targeting the blood flow changes directly associated with the neuronal activity, we expect that fASL activation would be in closer proximity to the actual neuronal sites compared to BOLD activation.
MATERIALS AND METHODS
Our experiments followed international guidelines and were approved by the local ethics committee. Twenty-eight right-handed healthy volunteers (14 women; 18–56 years of age; mean age, 33 years) were enrolled in this study and gave informed consent for their participation. All subjects had normal or corrected visual acuity and had no psychiatric or neurologic symptoms.
Experimental Design
Subjects underwent an MR imaging session (Magnetom Trio 3T; Siemens, 32-channel-head coil) with motor and somatosensory tasks, followed by a neuronavigated TMS examination on a different day (1–7 days later).
The motor task consisted of seven 35-second alternations between hand-clenching and rest. The somatosensory stimulation included ten 35-second blocks of rest and stimulation, during which the right or left thumb received nonpainful air wisps at 2.14 Hz using MR imaging–compatible pneumatic equipment. Participants were asked to look at a fixation cross during rest periods. Hand order was counterbalanced across participants.
During tasks, BOLD imaging and fASL were acquired simultaneously, with a dual-echo pseudocontinuous ASL sequence having the following parameters: TR = 3500 ms, TE1/TE2 = 10/25 ms, label duration = 1500 ms, post-labeling delay = 1000 ms, FOV = 205 × 205 mm, 3-mm-thickness slices with a 0.6-mm gap, in-plane resolution = 3.2 × 3.2 mm, 20 slices. The FOV covered the top half of the brain, and the labeling plane was positioned, with the help of a sagittal angiography sequence, 14 cm below the center of the image slab perpendicular to the carotids. Structural imaging included a high-resolution 3D T1 (multiecho MPRAGE: TR = 2530 ms, TI = 1100 ms, TE1/TE2/TE3/TE4 = 1.64/3.5/5.36/7.22 ms, 1-mm isotropic).
Individual MR imaging was used for neuronavigated TMS to map the motor cortex associated with both hands. TMS recordings were performed using the Navigated Brain Stimulation System from Nextim (https://www.nexstim.com/), following standard stimulation guidelines.35 Two pairs of electrodes were placed on the abductor pollicis brevis and the first dorsal interosseus muscles on each hand, and a ground-reference electrode was placed on the right wrist. Motor-evoked potentials were recorded throughout the entire stimulation. The anatomic hotspot in the precentral gyrus was visually identified for each hemisphere. The resting motor threshold for the first dorsal interosseus muscles was found by delivering single-pulse stimulations and recording the lowest intensity capable of eliciting 50-μV motor-evoked potentials in at least 50% of the stimulations, within a 20-ms latency range.36 The subject-specific stimulation intensity above the resting motor threshold (105%) was used for stimulation, while moving the coil medially and laterally around the predefined anatomic hotspot. Stimulation toward the postcentral and precentral sulci defined the boundaries of the somatotopy. Significant stimulation points associated with suprathreshold motor-evoked potentials (50-μV, peak-to-peak) were collected for further analysis.
Data Analysis
MR imaging data-preprocessing and analysis were performed using customized scripts in Matlab (MathWorks), SPM12 (IBM), and the open-source toolbox ASLtbx (https://www.cfn.upenn.edu/zewang/ASLtbx.php).37 Preprocessing consisted of realignment and coregistration to 3D T1 spatial smoothing using an isotropic Gaussian kernel (6 mm for BOLD imaging and 5 mm for fASL). For fASL data, label and control images were realigned separately and then coregistered to the 3D T1. Pair-wise subtraction between the label and control was considered to estimate the perfusion-weighted images.38 3D T1 was segmented, and a brain mask was created for further analysis.
Individual activation of BOLD imaging and fASL for the clenching task and the somatosensory stimulation was obtained with the independent FSL General Linear Model (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM) for each hand.
In clenching task, the threshold for significant BOLD activation was established at a family-wise error rate P < .001, with a requirement for a minimum extent of 30 contiguous voxels. Due to the known low SNR of fASL and because the total number of volumes was halved with respect to BOLD imaging, we considered P < .001 with a 10-voxel extent threshold for fASL. Activations associated with somatosensory stimulation are very focal and less robust than motor responses. Different thresholds were then explored to detect significant activations.
Systematic shifts of BOLD and fASL localization between them and versus TMS were analyzed at an individual level. For each activation map derived from BOLD imaging and fASL, coordinates of the global maximum of activiation (MAX) were collected in the individual MR imaging space. In parallel, the coordinates of the TMS point exhibiting the maximal motor-evoked potential amplitude for each hemisphere were considered and projected in the MR imaging individual space. To complement the information of the activation strength expressed by the MAX, we calculated the center of gravity (CoG)39 for each subject, hand, and technique. Indeed, the CoG allows accounting for the distribution of significant points, being defined as
 where i is the index of the point, L is the location (x, y, z), and V is the t value for fASL/BOLD maps and motor-evoked potential amplitude for the TMS at position Li.
where i is the index of the point, L is the location (x, y, z), and V is the t value for fASL/BOLD maps and motor-evoked potential amplitude for the TMS at position Li.
For fASL and BOLD imaging, CoG calculation was restricted to the cluster containing the MAX. For TMS, all significant points were included to account for the different neuronal excitability of the stimulated pericentral region.40
For each hand and alternatively for MAX and CoG, BOLD-fASL comparison was assessed in the subject's individual MR imaging space by calculating the Euclidean distance. Paired t tests were applied for all directions: left-to-right (x), posterior-to-anterior (y), and inferior-to-superior (z). Similarly, the proximity of fASL and BOLD imaging to TMS was assessed by paired t tests of the Euclidean distance.
Second-level analyses were also conducted after normalization into the Montreal Neurological Institute space to compare fASL and BOLD activation maps at the group level.
RESULTS
Two participants withdrew from the study due to claustrophobia during the MR imaging session. For 3 subjects, the coregistration between TMS and MR imaging failed due to an unresolved issue of the Nexstim software. Consequently, for the motor task, statistical assessment and group analysis between MR imaging modalities and TMS were conducted on 26 and 23 subjects, respectively. For the somatosensory stimulation, an additional participant was excluded due to incomplete task execution, and the analysis was performed on 25 subjects.
The Euclidean distances between fASL and BOLD imaging for each hand and in terms of MAX and CoG were significantly (P < .001) different from zero (Online Supplemental Data). The average values ranged between 10.12 and 16.61 mm, depending on the selection of MAX or CoG. Concordance of values (in 1 SD) was found between the right and left hand (Table 1).
Difference between fASL and BOLD in terms of Euclidean distance
This spatial difference was mainly driven by the inferior-superior (z) direction, where fASL was found significantly (P < .001) more inferior than BOLD imaging, following the anatomy of the central sulcus (Online Supplemental Data).
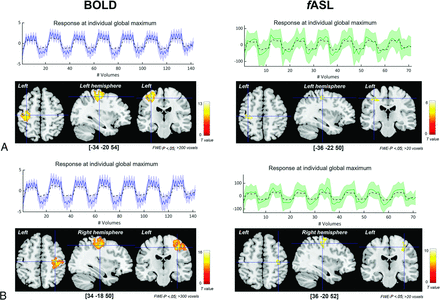
The distance along the z-axis varied from 6.94 to 12.91 mm, depending on the selection of MAX and CoG. Along the other anatomic directions, fASL localized between 0.62 and 3.10 mm more lateral than BOLD imaging (P < .05, except when considering the MAX during the clenching of the left hand) and between 0.41 and 2.93 mm more anterior than BOLD imaging (P < .05, except for the left clenching when the CoG was considered). The overview of the intra-MRI modality variability across participants is given in Figure 1 and in terms of the relative difference between fASL and BOLD imaging in Table 1.
Group analysis of clenching hand task. Results of the clenching of the right (A) and left (B) hand across the subjects are shown for BOLD (left side) and fASL (right side). Blue crosslines point at the global maxima of the group-level activation. Coordinates are reported in squared parentheses. Plots on top of each brain-view represent the BOLD (in blue) and fASL (green) timeseries of the individual global maxima. Straight lines and shaded areas indicate the means and the standard deviations evaluated at each timepoint across the subjects. The dashed black line represents the experimental design.
By comparing the Euclidean distances between fASL and TMS and between BOLD imaging and TMS, we observed a shift between 9.0 and 14.5 mm between MR imaging modalities and TMS (Table 2), in line with the literature.31,41 Moreover, only for the MAX and in the case of the left hand, was fASL significantly more proximal than BOLD to TMS (Online Supplemental Data and Table 2).
Results of the distances to TMS of fASL and BOLD
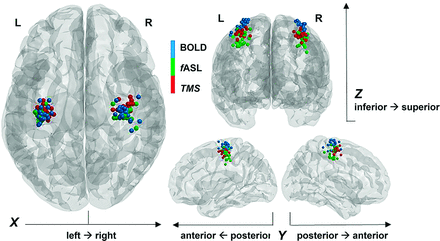
By investigating the difference along each orientation, we found that fASL was significantly more proximal to TMS than BOLD imaging along the inferior-superior direction (P < .001) for MAX in each hand condition and additionally along the left-right for the CoG (P < .05) in the left hand condition, as shown in the Online Supplemental Data. The shift among the 3 different mapping techniques can be observed in the Figure 2, which depicts the CoG of each hand, subject, and technique, projected on a Montreal Neurological Institute template.
Representation of CoG on MNI template. For each healthy participant, blue and green spheres are positioned on the MNI coordinates of the CoG for BOLD and fASL activations during the clenching motor task for each hand. The red spheres correspond to the MNI coordinates of the points with the highest motor evoked potential during TMS for each hand.
For the somatosensory stimulation of the thumb, though a flexible threshold of P < .001 was chosen, it was difficult to select the most representative localization between the MAX or secondary local maxima even for the BOLD imaging. For fASL, in which a threshold of P < .01 was used, this choice was even more difficult. To avoid localization bias by visual inspection, we excluded the possibility of relying on a statistical comparison at the level of coordinates of activation at the individual level. However, at the group level, the comparison between MAX showed that fASL localized more medially, anteriorly, and deeper than BOLD imaging (Online Supplemental Data).
DISCUSSION
This work assessed the role of fASL as a complementary or alternative solution to BOLD imaging for mapping the eloquent cortex. We selected motor and somatosensory tasks and reviewed the shift between fASL and BOLD imaging in a cohort of healthy participants. Instead of deriving the BOLD signal from ASL or considering a separate BOLD session,26,30,31 we used a simultaneous BOLD-fASL acquisition.27⇓-29,32,33 Additionally, we addressed the localization specificity between the 2 MR imaging modalities, while comparing motor task results with the hand somatotopy depicted by TMS (ie, TMS assumed as ground truth).
TMS is known to noninvasively identify the ω-shaped hand knob with millimeter resolution,42 demonstrating high spatial concordance with intraoperative and direct cortical stimulation.43
Selection of Experimental Paradigms
Given the extent of hand representation in the human brain,44 know from 1937,45 we used well-established hand sensorimotor stimulations to activate the primary motor (M1) and sensory (S1) cortices. In fMRI studies, hand-clenching is used for mapping M1, specifically the Rolandic region.46 Similarly, cutaneous low-threshold vibration on the fingertips reproduces the functional organization of S1.10,47 Brain activated regions have been compared across different imaging techniques. In the hand motor task, fMRI showed a more posterior and lateral localization with respect to electrical source imaging.48 The posterior shift has been confirmed between fMRI and motor TMS.9 For pneumatic stimulation, fMRI activated the central gyrus more laterally (8–20 mm) with respect to electromagnetic techniques.10,48,49
In our cohort, we confirmed that during hand-clenching, BOLD imaging localized more posteriorly and laterally than fASL for both hands (Table 1 and the Online Supplemental Data). For somatosensory stimulation, we found fASL more anterior than BOLD imaging for both thumbs and fASL more medial than BOLD imaging only for the left thumb. Most interesting, the Montreal Neurological Institute coordinates of the MAX were comparable with the results obtained in a previous work (Online Supplemental Data and Lascano et al10). Although derived from a group-level analysis, compared with BOLD imaging, fASL in the somatosensory task seems to share characteristics with electrical source imaging, leading to the hypothesis that fASL could be a more direct measure of spiking neurons. An fASL shift toward the midline with respect to BOLD imaging could be ascribed to the digit-specific somatotopy, which attributes a more medial location to the thumb, which, in our case, received the stimulation.42
Influence of Cerebral Vasculature
Across studies, the localization distance between BOLD-fMRI and other modalities during motor and somatosensory tasks is variable (10–30 mm).10,49
In patients with brain tumors, though there is a good concordance between preoperative BOLD-fMRI with intra- or extraoperative electrocortical stimulation, some localization discrepancies have been observed.50,51 The different origins of electrocortical and hemodynamic signals may affect the localization as well as cerebrovascular characteristics. Quantitative assessment of cerebral perfusion with ASL allowed overcoming the influence of the venous blood of BOLD imaging.
In our cohort, activations in somatosensory tasks showed a systematic anterior shift of fASL compared with BOLD for both hands. This finding could highlight the specificity of fASL in localizing arterial blood changes and the limitation of BOLD imaging affected by draining veins. Notably, the somatosensory area possesses a rich venous system, including the middle part of the anastomotic vein of Trolard, which runs into the postcentral sulcus, draining the adjacent cortex and the Rolandic vein, anterior to the vein of Trolard, which irrigates the pre- and postcentral sulci.52
The Choice of the Threshold
In fMRI localization, the spatial extent of the results depends on the thresholding procedure (ie, P value and minimum cluster size). For hand-clenching, we opted for a conservative threshold and reproduced and confirmed the findings of similar work.31 Somatosensory stimulation required a more flexible approach.
In addition, the choice of the “most representative” activation point is debatable.39,53 While the MAX seems less representative than the center of the mass and the CoG, it is less affected by thresholding. We analyzed our data both in terms of MAX and CoG, providing concordant intermodality results on both the strength and the distribution of activations. Notably, CoG was calculated for the first activation cluster (ie, containing the MAX). Thus, we restricted the analysis to the area around the central sulcus by avoiding central and contralateral coactivated regions, observed in some subjects.
TMS as Ground Truth
TMS was used to compare fASL and BOLD localizations of the hand motor task. As opposed to previous studies using the maximal fMRI activation as a reference point for TMS stimulation,31 in our study, the TMS operator was blinded to fMRI results and mapped the motor region using a structural MR imaging–navigated approach.
In line with previous studies, we reproduced the shift between fASL and BOLD imaging,26,31,54 along the inferior-to-superior and posterior-to-anterior axes. Moreover, we found that fASL was more proximal to TMS than BOLD imaging along the inferior-superior and mediolateral axes.
Limitations and Perspectives
The accuracy of our results should be interpreted in light of the intrinsic limitations of ASL, characterized by low spatial and temporal resolution. This limitation can be explained by the low SNR of the perfusion signal, which depends on the scarce tissual microvascularity in the voxel volume and the decay of the relaxation time during postlabeling delay.55
The spatial accuracy of fASL demonstrated in this work would be beneficial in patients with a potential modification of the neurovascular coupling. This is the case in patients with brain tumors, cerebral arteriovenous malformations, epileptic lesions, or stroke. In such instances, the alteration of the cerebral vascularity can, indeed, induce false-negative BOLD activations on the definition of functional areas.
CONCLUSIONS
In the current study, we investigated the spatial accuracy of fASL in delineating functional brain regions. In a cohort of 26 healthy subjects, using hand sensorimotor tasks, we found significant shifts between fASL and the standard BOLD imaging along all brain directions, and we observed that fASL targeted the sulci anatomy better than BOLD imaging. Moreover, the comparison with TMS showed that fASL is more proximal than BOLD imaging to the areas excited by stimulation and can, therefore, be a more direct representation of neuronal spiking. These findings make fASL a valid alternative or complement to the standard functional imaging, particularly in patients with potentially altered neurovascular coupling, which could bias localization of the eloquent cortex.
ACKNOWLEDGMENTS
This work was performed using the imaging platform at the Brain Behaviour Laboratory (BBL), University of Geneva, which received the financial support of Swiss National Science Foundation (R'Equip grant, 326030_205728). The authors thank the technical staff of the BBL and of the CIBM Center for Biomedical Imaging for their support. We acknowledge the receipt of the dual-echo pseudocontinuous arterial spin-labeling sequence from the University of Southern California's Stevens Neuroimaging and Informatics Institute. We also acknowledge The Regents of the University of California, on behalf of its Los Angeles campus, as the source of portions of the licensed technology. We acknowledge the Clinical Research Center, University Hospitals and Faculty of Medicine in Geneva.
Footnotes
This study was supported by the Louis-Jeantet Foundation.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 11, 2023.
- Accepted after revision August 28, 2023.
- © 2023 by American Journal of Neuroradiology