Abstract
BACKGROUND AND PURPOSE: Data on SAH after M2 mechanical thrombectomy are limited. We aimed to determine the prevalence of sulcal SAH after mechanical thrombectomy for M2 occlusion, its associated predictors, and the resulting clinical outcome.
MATERIALS AND METHODS: The study retrospectively reviewed the data of patients with acute ischemic stroke who underwent mechanical thrombectomy for isolated M2 occlusion. The patients were divided into 2 groups according to the presence of sulcal SAH after M2 mechanical thrombectomy. Angiographic and clinical outcomes were compared. Multivariable analysis was performed to identify independent predictors of sulcal SAH and unfavorable outcome (90-day mRS, 3–6).
RESULTS: Of the 209 enrolled patients, sulcal SAH was observed in 33 (15.8%) patients. The sulcal SAH group showed a higher rate of distal M2 occlusion (69.7% versus 22.7%), a higher of rate of superior division occlusion (63.6% versus 43.8%), and a higher M2 angulation (median, 128° versus 106°) than the non-sulcal SAH group. Of the 33 sulcal SAH cases, 23 (66.7%) were covert without visible intraprocedural contrast extravasation. Distal M2 occlusion (OR, 12.04; 95% CI, 4.56–35.67; P < .001), superior division (OR, 3.83; 95% CI, 1.43–11.26; P = .010), M2 angulation (OR, 1.02; 95% CI, 1.01–1.04; P < .001), and the number of passes (OR, 1.58; 95% CI, 1.22–2.09; P < .001) were independent predictors of sulcal SAH. However, covert sulcal SAH was not associated with an unfavorable outcome (P = .830).
CONCLUSIONS: After mechanical thrombectomy for M2 occlusion, sulcal SAH was not uncommon and occurred more frequently with distal M2 occlusion, superior division, acute M2 angulation, and multiple thrombectomy passes (≥3). The impact of covert sulcal SAH was mostly benign and was not associated with an unfavorable outcome.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- CA
- contact aspiration
- EVT
- endovascular treatment
- GRE
- gradient recalled-echo
- ICH
- intracerebral hemorrhage
- MT
- mechanical thrombectomy
- mTICI
- modified TICI
- SR
- stent retriever
- SS
- sulcal SAH
Since the publication of a recent endovascular treatment (EVT) trial in 2015,1 many studies have demonstrated the feasibility and safety of mechanical thrombectomy (MT) for treating patients with MCA M2 occlusion, which is a medium-vessel occlusion.2-6 Accordingly, acute ischemic stroke (AIS) due to M2 occlusion has been increasingly treated with EVT despite the lack of clear guideline-based recommendations.7 However, the anatomic features of M2, including smaller diameters, tortuous vessels, and thinner vessel walls could be linked to poor accessibility and high periprocedural complication rates.8
SAH is one of the most concerning periprocedural complications after MT for AIS according to neurointerventionalists.9-11 More distal and narrower M2 vessels could particularly lead to iatrogenic SAH; however, to date, its related procedural factors have not been fully elucidated. In our preliminary experience, sulcal SAH (SS), which was undetected during M2 MT, was observed on postprocedural imaging. Given the technical challenges and lesser severity of M2 stroke, balancing the potentially increased risk of periprocedural complications and the clinical benefit of MT for M2 occlusion is essential.12 However, the clinical impact of SS after M2 MT remains largely unknown.
This study aimed to investigate the frequency and angiographic predictors of SS. We also investigated the clinical impact of SS, focusing on covert SS, which is angiographically occult but is detected by postprocedural imaging in patients who undergo MT for isolated M2 occlusion.
MATERIALS AND METHODS
Patients
This study was approved by the local institutional review board of the Seoul National University Bundang Hospital, and the requirement for written informed consent was waived because of the retrospective nature of this study.
This retrospective analysis included all consecutive patients with AIS who underwent EVT between January 2013 and July 2021 at our stroke center. The inclusion criteria for the study were as follows: 1) time from symptom onset to groin puncture ≤24 hours; 2) primary isolated MCA M2 segment occlusion visible on CT or MR angiography; 3) baseline NIHSS score, ≥2 points; 4) history of immediate postprocedural and follow-up CT or MR imaging; and 5) stent retriever (SR), contact aspiration (CA), or CA with an SR as the primary treatment. The exclusion criteria of this study were as follows: 1) tandem or multiple occlusions, 2) prestroke mRS score >2, and 3) insufficient data or poor image quality. This study defined the M2 segment as the MCA segment from the genu to the circular sulcus of the insula; early MCA branches arising from the M1 segment before the genu were not considered.2,3
Endovascular Treatment
All procedures were performed by 3 experienced neurointerventionalists (C.J., S.H.B., and J.Y.K.) using the femoral artery approach with the patient under local anesthesia or conscious sedation. Unless technically infeasible, a balloon-guide catheter was routinely used. The choice of the first-line technique was left to the discretion of the treating operator. All included patients were treated with an SR alone, CA alone, or CA combined with an SR. If successful reperfusion was not achieved with the initially selected first-line MT despite multiple attempts, rescue therapy was performed by switching to the other strategy.
Thrombectomy with SR was performed using an SR device (Solitaire 4 × 20 mm, Covidien; Trevo 3 × 20/4 × 20 mm, Stryker; or Aperio 3.5 × 28 mm, Acandis). CA thrombectomy was performed using an aspiration catheter (3MAX or 4MAX; Penumbra) or an intermediate catheter (Sofia 5F; MicroVention; or AXS Catalyst 6, Stryker) alone or in combination with an SR. The details of the conventional MT technique have been described previously.9,13
Data Collection and Outcome Measures
The primary outcome was the occurrence of SS after M2 mechanical thrombectomy. Patients were divided into SS and non-SS groups according to the presence of SS after M2 MT. SS was diagnosed by a hypointense and hyperintense lesion in the subarachnoid space, which was located in the Sylvian fissure or cerebral sulci on gradient recalled-echo (GRE) T2* and FLAIR sequences or via a persistent hyperdense lesion on serial noncontrast CT.9,14 The interpretation of SAH with a GRE sequence took precedence over the CT scan because the former can distinguish true SAH from contrast leakage.14 The diagnosis of SS with CT was performed if there was persistent sulcal hyperdensity on serial CT scans to exclude the contrast leakage. Regarding the institutional protocol, immediate postprocedural MR imaging or CT was performed, and follow-up MR imaging was routinely performed at our center 3–5 days after EVT. Additionally, secondary SAH that was not detected in the immediate postprocedural CT or MR imaging but was accompanied by hemorrhagic transformation was not included in the SS group.
Patients with SS were further classified into overt or covert SS subgroups according to the presence of intraprocedural contrast extravasation. Overt SS was defined as diffuse or confluent SAH with apparent intraprocedural contrast extravasation during EVT on DSA. Conversely, covert SS was defined as minimal, isolated SAH identified only on postprocedural MR imaging or CT, without intraprocedural contrast extravasation (Figure). All angiographic and imaging findings were independently checked by 2 neurointerventionalists (D.Y.K. and S.H.B.) in consensus.
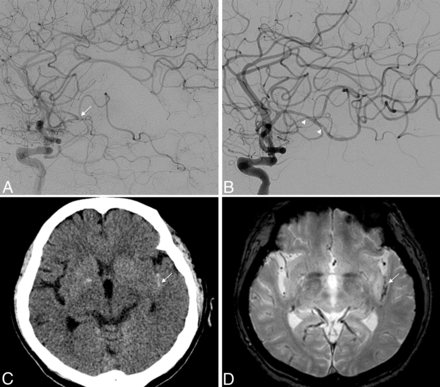
Illustrative case showing covert SS. A patient in her 60s presented with aphasia and right-sided weakness. A, A left ICA lateral angiogram shows occlusion of distal M2 segment (arrow). B, After 3 attempts of thrombectomy using a Trevo 3 × 20 mm (not shown), successful reperfusion was achieved. Note that vasospasm is revealed (arrowheads) without definite contrast extravasation. M2 angulation is measured as 130° on the final angiogram. C, A noncontrast CT scan obtained 1 day after thrombectomy shows a small amount of hyperdense lesions (arrow) in the left Sylvian fissure. D, Axial GRE MR imaging performed 3 days after thrombectomy reveals an apparent hypointense signal lesion in the left Sylvian fissure (arrow), consistent with SAH.
M2 angulation was defined as a total calculated vessel angle that was the sum of the calculated M1–M2 and M2–M2´ angles.15 The M1–M2 angle was defined as the angle from the M1 segment as it turns superiorly (at the M1–M2 junction) to become the M2 segment into which the thrombectomy device is positioned as the vessel courses toward the circular sulcus. M2–M2´ was defined as the angle between the just proximal and distal M2 segment of clot. Each calculated M1–M2 and M2–M2´ angle was obtained by subtracting the measured angle from 180°. Thus, a higher number of M2 angulations indicates greater vessel curvature. For the assessment of vessel angle, the best vessel contrast was selected from the final angiogram without a thrombectomy device in situ to avoid straightening the vessel anatomy. We measured the angles on the frontal projection of 2D DSA from the final angiogram using a PACS tools for angle measurement (Online Supplemental Data).
The demographic information included age, sex, and vascular risk factors (hypertension, diabetes, dyslipidemia, current smoking, coronary artery disease, atrial fibrillation, and previous stroke history). Stroke and treatment information, such as occlusion site mechanism (thromboembolic, in situ thrombosis, or unknown), IV tPA use, admission NIHSS score, baseline ASPECTS, M2 divisions (superior, inferior, or both), and proximal/distal M2 location, were collected. The proximal and distal M2 segments were distinguished by the line delineating the midheight of the insula.
According to the modified TICI (mTICI) scale, we reported successful and complete reperfusion rates (defined as mTICI 2b–3 and mTICI 3, respectively). The number of thrombectomy passes was counted, and the outcomes and complications were analyzed. The first-pass effect was defined as achieving complete reperfusion with a single thrombectomy device pass. A favorable clinical outcome was defined as a 90-day mRS score of 0–2.
Safety outcomes included procedural (perforation, dissection, vasospasm, and distal embolism) and hemorrhagic complications. Vessel perforation was defined as frank angiographic contrast extravasation that occurred during the procedure. Distal embolism was defined as fragmentation of a primary clot downstream of the primary occlusion and embolization into a new territory. Intracerebral hemorrhage (ICH) was classified according to the European-Australasian Acute Stroke Study classification, and symptomatic intracerebral hemorrhage was defined as any hemorrhage associated with an NIHSS score increase of ≥4 points within 24 hours.16 Neurologic deterioration associated with SAH was defined as a ≥4-point increase in the NIHSS score assessed 24 hours after MT that was not attributable to any other cause.10,17
Statistical Analysis
The differences in the baseline characteristics as well as the procedural and clinical outcomes were compared between patients with and without SS. The Pearson χ2 test or the Fisher exact test was used for categoric variables, and the Student t test or Mann-Whitney U test was used for continuous variables. A binary logistic regression analysis was performed to determine the predictors of SS. Finally, the association between covert SS and 90-day clinical outcomes was investigated only in patients without intraprocedural contrast extravasation (overt SS). Variables with a P value < .10 from univariate analysis and those considered clinically relevant were included in a multivariate model. All statistical analyses were performed using R version 3.5.1 (http://www.r-project.org/). Statistical significance was set at P < .05.
RESULTS
A total of 1502 patients with AIS underwent EVT between January 2013 and July 2021. Among these, 236 patients who met the inclusion criteria were initially enrolled. Of these, 27 patients were excluded for the following reasons: 1) tandem or multiple occlusions (n = 11); 2) prestroke mRS score of >2 (n = 6); and 3) insufficient data or poor image quality (n = 10). Finally, 209 patients (mean age, 69 [SD, 12.4] years; 110 men [56.9%]) with primary isolated M2 occlusions qualified for the final analysis. Of the enrolled 209 patients, SS was observed in 33 (15.8%) patients; the remaining 176 patients (84.2%) were assigned to the non-SS group.
Baseline and Procedural Characteristics
The baseline characteristics of the SS and non-SS groups are presented in Table 1. The median NIHSS score at admission was 10 (interquartile range, 7–15). The involved sites were the proximal M2 (69.9%) and the superior division (46.9%). The SS group more frequently had distal M2 (69.7% versus 22.7%, P < .001) and superior division (63.6% versus 43.8%, P = .036) occlusion compared with the non-SS group. Age, vascular risk factors, etiology of the occlusion, intravenous tPA, and admission ASPECTS were comparable between the 2 groups.
Baseline characteristics between sulcal SAH versus non-sulcal SAHa
The treatment and clinical outcomes are summarized in the Online Supplemental Data. Thrombectomy with an SR was most often used as the first-line treatment (71.8%). Overall, successful recanalization was achieved in 85.2% of the patients. The SS group showed a lower rate of CA thrombectomy (0% versus 11.9%, P < .001), higher M2 angulation (median 128° versus 106°, P < .001), and a larger number of passes (median, 3 versus 1; P < .001) than the non-SS group. Additionally, the SS group showed a longer procedural time (median, 48 versus 28 minutes; P < .001), a lower rate of successful reperfusion (57.6% versus 90.3%, P < .001), and a lower first-pass effect (0% versus 29.0%, P = .001). There were no significant differences in the rates of favorable clinical outcome or mortality between the 2 groups. The incidence of hemorrhagic complications was not significantly different between the 2 groups.
Characteristics of Sulcal SAH
Thirty-three patients had SS, of whom 10 (30.3%) had overt SS with intraprocedural contrast extravasation, whereas the remaining 23 (69.7%) patients had covert SS with angiographically occult SAH detected on postprocedural imaging (Table 2). Seven of the 10 patients with overt SS underwent emergent coil embolization, whereas contrast extravasation spontaneously ceased in the remaining 3 patients during the procedure. Covert SS was observed only in the ipsilateral Sylvian fissure (n = 14, 60.9%) and in the Sylvian fissure with other sulci (n = 9, 39.1%) (Fig 1). Of the 23 patients with covert SS, only one (4.3%) was symptomatic with neurologic deterioration, resulting in a prevalence of 0.48%. Conversely, patients with overt SS showed a higher rate of neurologic deterioration (40% versus 4.3%; P = .021) and unfavorable outcome (70% versus 47.8%; P = .283) than those with covert SS.
Characteristics of SSa
Predictors of Sulcal SAH
In the univariate analysis, procedural time, distal M2, superior division, M2 angulation, and number of passes were associated with SS (Table 3). In the multivariable analysis, distal M2 occlusion (OR, 12.04; 95% CI, 4.56–35.67; P < .001), superior division (OR, 3.83; 95% CI, 1.43–11.26; P = .010), M2 angulation (OR, 1.02; 95% CI, 1.01–1.04; P < .001), and number of passes (OR, 1.58; 95% CI, 1.22–2.09; P < .001) were independent predictors of SS. Additionally, distal M2 occlusion (OR, 8.53; 95% CI, 2.98–27.33; P < .001), superior division (OR, 3.86; 95% CI, 1.30–13.00; P = .020), M2 angulation (OR, 1.02; 95% CI, 1.01–1.03; P = .005), and number of passes (OR, 1.60; 95% CI, 1.21–2.13; P < .001) were also significantly associated with covert SS (Online Supplemental Data).
Multivariable analysis for predictors of SS
Impact of Covert SS on Clinical Outcomes
In the subgroup analysis excluding 10 patients with overt SS, age (OR, 1.11; 95% CI, 1.05–1.18; P < .001), atrial fibrillation (OR, 0.33; 95% CI, 0.11–0.90; P = .037), admission NIHSS score (OR, 1.15; 95% CI, 1.05–1.26; P = .002), baseline ASPECTS (OR, 0.53; 95% CI, 0.32–0.83; P = .009), and parenchymal hematoma (OR, 21.93; 95% CI, 2.31–50.77; P = .015) were predictors of unfavorable outcome in the multivariate logistic regression analysis. However, covert SS was not significantly associated with unfavorable outcome (P = .830) (Online Supplemental Data).
DISCUSSION
In the current study, SS after MT for M2 occlusion was not uncommon, and two-thirds of SS cases were covert SS, which were angiographically occult but revealed by postprocedural imaging. In addition, several procedural factors including distal location, superior division, acute vessel angulation, and multiple passes (≥3) were independently associated with the occurrence of SS. Although common, SS has a non-notable impact and is not associated with unfavorable clinical outcome.
To the best of our knowledge, this is the first study to show the prevalence of SAH as well as related procedural factors and clinical impact in patients undergoing M2 thrombectomy. Although there have been several studies on MT for M2 occlusion, they merely reported either the rate of symptomatic ICH or safety outcomes that focused on intraprocedural SAH or perforation.2,4,5,12,18-21 However, the present study attempted to assess not only overt SS but also covert SS and investigated procedural predictors of SS. The current study indicates that SS following M2 thrombectomy is relatively common, and distally located, superior division, acute-angle M2 occlusion with multiple thrombectomies is likely to result in SS.
The incidence of SAH after MT ranges from 0.5% to 24% according to previous reports,9-11,15,22-25 and in our cohort, the observed incidence was 15.8%. In previous M2 studies, SAH after M2 thrombectomy ranged from 0.9% to 7.7%,1-5,8,18,19,23 which was still lower than our data (15.8%). However, if the definition of SAH is restricted to the procedural angiographic findings, intraprocedural SAH was 4.8% in this study, showing similar or lower rates of SAH than in other studies. In addition, the incidence of SAH in previous studies might have been under-reported because of the lack of MR imaging or follow-up CT. According to the protocol at our center, most patients with AIS who underwent MT also underwent MR imaging within 3 days after EVT. Therefore, postprocedural MR imaging was available for 184 (88.0%) of the enrolled patients. As described in Table 2, in addition, 71% of angiographically occult SAH cases were found by MR imaging; thus, a higher proportion of SAH might be attributable to the application of the GRE sequence, which offers higher sensitivity than CT alone in diagnosing SAH.
The clinical consequences of SAH differ according to the type of SAH and the co-occurrence of ICH.24,25 While isolated SAH after MT does not influence long-term clinical outcomes, the presence of diffuse SAH, including that associated with concomitant parenchymal hematoma, reduces the chances of clinical recovery. Qureshi et al25 showed that the rates of independent functional outcome were lower among subjects with SAH with intraparenchymal hemorrhage or other ICH but not in subjects with isolated SAH. Renú et al24 recently reported that the post-MT diffuse subarachnoid hyperattenuation pattern was mostly composed of blood extravasation and emerged as a biomarker of an increased risk of poor outcome and death. In line with these studies, covert SS was not associated with unfavorable outcome, whereas overt SS showed a trend toward unfavorable outcome.
The elevated SAH risk due to distal location and acute M2 angulation in the current study might be explained by several possible mechanisms: First, a more curved, distal location with thinner vessel walls causes difficulties in procedural accessibility and a higher risk of microguidewire perforation and tearing of tiny arterioles. Second, in the distally located M2 segment, because these vessels have a small normal diameter, any reduction in vessel caliber would result in greater friction and traction forces during MT than in larger proximal vessels. Third, acute M2 angulation increases friction between the vessel wall and the device, with increased vessel stretching during stent retrieval.26 As noted in our study, Ng et al15 found a trend toward postprocedural SAH occlusion in patients with extreme MCA angulation and distal device positioning. Lee et al11 also reported that the distal location of vessel occlusion was associated with a higher probability of SAH.
A higher number of MT passes was associated with SS in this study. Previous studies have shown that a greater number of thrombectomy device passes increases the prevalence of SAH, supporting our results.10,11,15 This outcome may occur because additional passes of the SR cause repeat traction injury to small perforating branches or venules. In addition, multiple passes of MT necessitate repeat guidewire probing of the occluded vessel, which increases the potential for unrecognized microguidewire perforation or dissection. Microperforation may not result in overt contrast extravasation on DSA or immediate bleeding if the vessel remains occluded or if the vessel immediately develops vasospasm. Subsequent recanalization of an occluded vessel with occult microperforation or dissection may lead to delayed occult SAH.15
Most interesting, there was no occurrence of SS in patients treated with CA alone as the primary MT method for M2 occlusion. In contrast, 78.8% of patients in the SS group were treated with an SR alone. Similar to our findings, Renieri et al21 recently reported that SR and combined techniques were associated with higher rates of intraprocedural SAH. Theoretically, SRs result in more extensive endothelial injury than aspiration catheters during stent retrieval because they exert a continuous radial action against the vessel wall.27,28 Additionally, it has been suggested that an SR exerts traction forces applied to the vessel, and deformation of the adjacent anatomy causes stretching and rupture of small arterioles or venules.29 Because distal M2 branches typically have a small diameter (≤2.0 mm), a higher traction force might be required during SR thrombectomy, which increases the likelihood of stretch injury to M2 perforating arterioles, and this phenomenon might be exacerbated by the greater M2 angulation compared with a proximal and smaller M2 angulation.15,27
In our study, although clinical consequences were different between overt and covert SS, distal M2 occlusion and acute M2 angulation were associated with both overt and covert SS. Therefore, when planning a procedural strategy, identifying these angiographic findings is important, and when the performing M2 thrombectomy under these anatomic conditions, a tailored thrombectomy technique as well as delicate microguidewire navigation is required to reduce SS occurrence.
This study has several limitations. First, inherent selection bias was inevitable because of the nonrandomized and retrospective study design. In particular, it is challenging to deliver a thromboaspiration catheter to the face proximal to the clot, especially in the distally located or curved M2, and this issue may influence the selection of first-line MT devices. Thus, careful interpretation of the results is required. Second, the sample size may not have been large enough to show statistical differences between the subgroups of this cohort from a single center. Most important, the sample size of the SS subgroups was very small, thus hampering appropriate analysis of their association with clinical outcome variables. Additionally, the application rate of CA was low (10.0%), and the differential effect derived from the use of the device on SS was not sufficient for assessment. Third, the wide range of the study period, including the potential effect of advancements in endovascular techniques and operator skill on treatment outcomes, is another potential limitation of this study.
CONCLUSIONS
After MT for M2 occlusion, SS was not uncommon and occurred more frequently with distal M2 occlusion, superior division, acute M2 angulation, and a higher number of thrombectomy passes. The clinical course of SS was different according to the type of SS. The impact of covert SS was mostly benign and was not associated with unfavorable outcome, whereas overt SS was symptomatic.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received April 7, 2022.
- Accepted after revision June 17, 2022.
- © 2022 by American Journal of Neuroradiology