Abstract
BACKGROUND AND PURPOSE: ICA-selective MRA using a pencil beam presaturation pulse can accurately visualize anterior communicating artery flow. We evaluated the impact of anterior communicating artery flow on the perioperative hemodynamic status and new ischemic lesions after carotid revascularization.
MATERIALS AND METHODS: Eighty-three patients with carotid artery stenosis were included. We assessed anterior communicating artery flow using ICA-selective MRA. The preoperative hemodynamic status was measured using SPECT. We also measured the change in regional cerebral oxygen saturation after temporary ICA occlusion. New ischemic lesions were evaluated by DWI on the day after treatment.
RESULTS: Anterior communicating artery flow was detected in 61 patients, but it was not detected in 22 patients. Preoperative cerebrovascular reactivity was significantly higher in patients with (versus without) anterior communicating artery flow with a mean peak systolic velocity of ≥200 cm/s (39.6% [SD, 23.8%] versus 25.2% [SD, 16.4%]; P = .030). The decrease in mean regional cerebral oxygen saturation was significantly greater in patients without (versus with) anterior communicating artery flow (8.5% [SD, 5.6%] versus 3.7% [SD, 3.8%]; P = .002). New ischemic lesions after the procedure were observed in 23 patients. The multivariate logistic regression analysis revealed that anterior communicating artery flow (OR, 0.07; 95% CI, 0.012–0.45; P = .005) was associated with new ischemic lesions.
CONCLUSIONS: The absence of anterior communicating artery flow influenced the perioperative hemodynamic status in patients with carotid stenosis and was associated with an increased incidence of new ischemic lesions after carotid revascularization.
ABBREVIATIONS:
- AcomA
- anterior communicating artery
- BeamSAT
- pencil beam presaturation
- CAS
- carotid artery stenting
- CEA
- carotid endarterectomy
- CVR
- cerebrovascular reactivity
- PSV
- peak systolic velocity
- rSO2
- regional cerebral oxygen saturation
Collateral flow via the circle of Willis plays an important role in hemodynamic status in patients with steno-occlusive ICA disease, and its presence is associated with favorable outcomes.1⇓⇓-4 In addition, the development of collateral flow via the circle of Willis is important for maintaining blood flow during temporary ICA occlusion in carotid endarterectomy (CEA) and carotid artery stenting (CAS).5 Previous studies have demonstrated that reduced cerebrovascular reactivity (CVR) and reduced cerebral perfusion during temporary ICA occlusion predict new ischemic lesions on MR imaging after CEA and CAS.6⇓-8
Conversely, the development of collateral flow via the circle of Willis is related to arterial morphologic features in the circle of Willis, such as the presence of anterior communicating artery (AcomA) flow or posterior communicating artery flow.9,10 The collateral route via the AcomA is considered particularly important.11 However, the relationship between the configuration of the circle of Willis and new ischemic lesions on MR imaging after carotid revascularization has been poorly investigated.12 In particular, no reports have focused on the relationship between the presence of AcomA flow and new ischemic lesions on MR imaging after carotid revascularization.
Pencil beam presaturation (BeamSAT) pulse, which is a new MR imaging method, enables suppression of the flow signal of a target vessel using 3D TOF-MRA and allows ICA-selective MRA to be performed.13,14 Previously, we reported that ICA-selective MRA using a BeamSAT pulse can accurately detect AcomA flow and predict ischemic intolerance to temporary ICA occlusion during CEA or CAS.14
As the degree of ICA stenosis progresses, cerebral perfusion pressure decreases. CBV increases in response to reduced cerebral perfusion pressure by dilation of intracranial cerebral vessels, leading to CBF preservation and a reduction in CVR.15 In contrast, collateral flow development is thought to preserve CBF and might contribute to the preservation of CVR.2 Accordingly, we hypothesized the following: In patients with cervical ICA stenosis, inadequate collateral flow via the circle of Willis due to the absence of AcomA flow leads to development of new ischemic lesions after carotid revascularization because of impaired CVR and reduced cerebral perfusion during temporary ICA occlusion.
The objective of this study was to validate this hypothesis and to evaluate the impact of AcomA flow on perioperative hemodynamic status in patients with carotid stenosis and new ischemic lesions on MR imaging after carotid revascularization. First, we investigated the association between the presence of AcomA flow on ICA-selective MRA and preoperative CBF and CVR on SPECT. Second, we examined the association between the presence of AcomA flow on ICA-selective MRA and regional oxygen saturation (rSO2) changes during temporary ICA occlusion. Third, we determined whether the absence of AcomA flow on ICA-selective MRA predicted development of new ischemic lesions on MR imaging after carotid revascularization.
MATERIALS AND METHODS
Patients
We prospectively recruited patients who were under consideration for either CEA or CAS and retrospectively reviewed the BeamSAT results of these patients. Between March 2015 and December 2020, eighty-three patients who underwent preoperative examinations before carotid revascularization were included. The inclusion criteria were carotid stenosis of ≥50% for symptomatic patients and stenosis of ≥60% for asymptomatic patients according to the criteria outlined in previous studies.16,17 Symptomatic patients were defined as patients who experienced amaurosis fugax, TIA, or stroke in the territory of the ipsilateral carotid artery within 6 months before entry. The institutional review board of Kobe University approved this study, and written informed consent was obtained from all patients.
MR Imaging Study
We performed MR imaging, including DWI, before and the day after the CEA or CAS, as well as MR imaging with the BeamSAT pulse before the procedure. No clinical signs or symptoms of a new ischemic event developed between the last DWI examination and the procedure.
DWI was performed using a 3T MR imaging scanner (Achieva; Philips Healthcare). The images were obtained using a spin-echo EPI sequence: TR, 3000 ms; TE, 48 ms; flip angle, 90°; sensitivity encoding, 3; FOV, 350 mm (rectangular FOV, 65%); matrix, 142 × 176 (reconstruction, 256 × 256); section thickness, 4 mm; gap, 1 mm; sections, 24–26; fat suppression, spectral presaturation with inversion recovery; b-value, 1000 s/mm2; number of excitations, 2; scan time, 1 minute 3 seconds.
ICA-selective MRA was performed using a 1.5T MR imaging scanner (Echelon Vega; Hitachi) and an 8-channel head coil, as previously reported.14 The 3D TOF-MRA parameters were as follows: TR, 23.0 ms; TE, 6.9 ms; flip angle, 20°; FOV, 230 mm; matrix, 512 × 200; section interval, 0.55 mm (after zero-fill interpolation); number of slices, 152; scan time, 4 minutes 50 seconds. For selective 3D TOF-MRA, BeamSAT pulse positioning was performed on a TOF source image from conventional MRA, and suppression of the flow signal in the region of the arteries covered by the BeamSAT pulse on 3D TOF-MRA was achieved. To depict the target artery, we suppressed other arteries with a 30-mm-diameter BeamSAT pulse.
ICA-selective MRA was performed as follows: When we adjusted the insertion direction of the BeamSAT pulse to penetrate and suppress the flow signals of the 3 major vessels (contralateral ICA and bilateral vertebral arteries) (Figs 1A, -B), only the target ICA flow signal remained, and ICA-selective MRA was performed (Fig 1C).
A, The BeamSAT pulse is positioned to cover the unilateral petrous portion of the ICA and bilateral vertebral arteries in an axial TOF source image obtained with conventional MRA (A). By adding the BeamSAT pulse to the unilateral ICA and bilateral vertebral arteries on 3D TOF-MRA (B), we performed ICA-selective MRA (C). The asterisk indicates BeamSAT.
AcomA Flow Evaluation
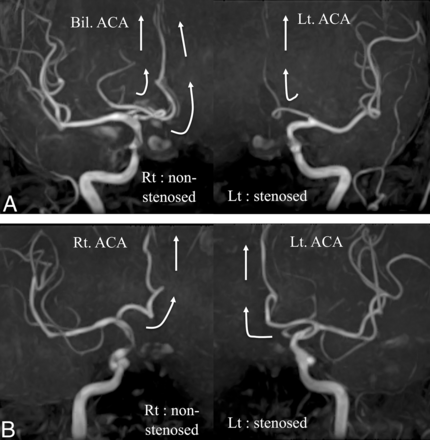
We performed bilateral ICA-selective MRA for AcomA flow evaluation for all subjects. The method used to evaluate AcomA flow using ICA-selective MRA has been reported previously.14 When the bilateral anterior cerebral arteries (distal to A2) were perfused from the unilateral ICA, they were classified into the AcomA (+) group (Fig 2A). When the anterior cerebral artery (distal to A2) on each side was perfused only from the ipsilateral ICA, they were classified into the AcomA (−) group (Fig 2B). The presence of AcomA flow was assessed by 2 observers blinded to the clinical information, both of whom are Japan Neurosurgical Society board–certified neurosurgeons.
A, A case with an AcomA. The bilateral anterior cerebral arteries are perfused from the ICA of the nonstenotic side. Only the ipsilateral anterior cerebral artery is perfused by the ICA of the stenotic side. B, A case without an AcomA. Each ICA perfuses only the ipsilateral anterior cerebral artery. White arrows show the direction of blood flow. Bil. indicates bilateral; Rt., right; Lt., left.
SPECT
All patients were scanned with a rotating dual-headed γ-camera with dynamic SPECT for 50 minutes (ECAM GMS7700; Toshiba Medical) before and the day after the operation. A dual-table autoradiographic method was used, which was developed for use with diffusible tracers to quantify CBF at rest and after pharmacologic stress from a single session of dynamic scanning with dual-bolus administration of N-isopropyl-[123I]-p-iodoamphetamine.18 The subjects were administered 2 doses of N-isopropyl[123I]-p-iodoamphetamine (111 MBq each) with a constant infusion for 1 minute at the beginning and at 30 minutes of dynamic SPECT imaging, and acetazolamide was injected 10 minutes before the second injection of N-isopropyl[123I]-p-iodoamphetamine. The SPECT scans (both resting and acetazolamide-challenged scans) started immediately after administration of N-isopropyl[123I]-p-iodoamphetamine. Arterial blood sampling was performed 10 minutes after the first N-isopropyl[123I]-p-iodoamphetamine administration. CBF quantification was performed using the quantitative SPECT/dual-table autoradiography method, which automatically and accurately corrects attenuated absorption and scattered radiation.19 We used the NEUROSTAT software library (http://128.95.65.28/∼Download/) for anatomic standardization of SPECT images. This software generates standardized 3D stereotactic surface projection data sets for individual patients. ROIs were automatically placed in both the cerebral and cerebellar hemispheres with a NEURO FLEXER (https://neuro-flexer.software.informer.com/) template.20 The mean CBF in the resting state and with the acetazolamide challenge was measured in the MCA territory ipsilateral to the carotid stenosis. Then, the CVR to the acetazolamide challenge was calculated as follows: CVR (%) = [(Acetazolamide-Challenged CBF – Resting CBF)/resting CBF] × 100.
Carotid Artery Sonography
All patients underwent carotid Doppler ultrasonography to observe the carotid artery and atherosclerotic plaque characteristics before the procedure. The peak systolic velocity (PSV) of the ICA and plaque characteristics, such as ulceration and calcification, was evaluated.
Surgical Procedures
Surgical procedures for CEA and CAS have been previously reported.14,21,22 In cases of CEA, patients were administered at least 1 antiplatelet agent for a minimum of 7 days before the procedure. CEA was performed with the patient under general anesthesia and somatosensory-evoked potential monitoring for selective shunt placement. In cases of CAS, patients were administered 2 antiplatelet agents for a minimum of 7 days before the procedure. CAS was performed with the patient under local anesthesia and general heparinization with embolic-protection devices.
rSO2 Monitoring
We monitored the rSO2 using a near-infrared spectroscopy oximeter (INVOS 5100C Cerebral Oximeter; Medtronic), as previously reported.14 Adhesive optode pad sensors were placed at the bilateral frontotemporal area. Monitoring of the rSO2 was started before anesthetic induction, and the rSO2 value was checked every 1 minute during the procedure. We measured the change in rSO2 before and after ICA clamping during external carotid artery occlusion for CEA and the change before and after distal ICA balloon inflation for CAS.
Statistical Analysis
Statistical analyses were performed using open-source software (R4.0.3; http://www.r-project.org). Descriptive statistics are presented as mean (SD) and were compared using the Welch 2-sample t test. The proportion of patients with each parameter was compared using the Fisher exact test. The relationships between patients’ baseline characteristics and new ischemic lesions on MR imaging were evaluated using the multivariate logistic regression model. The covariates included in the model were age, procedure (CEA or CAS), the presence of AcomA flow, the presence of ulcerated plaques, and preoperative CVR. These covariates were selected on the basis of previous literature and expert opinion.7,8,23⇓⇓⇓-27 The ORs of age and CVR are presented as estimated odds of outcome for a 1 year increase in age and a 1% increase in percentage. P values < .05 were considered statistically significant.
RESULTS
Association between AcomA Flow and Hemodynamic Status on SPECT
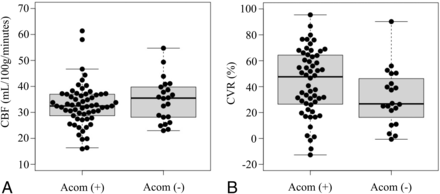
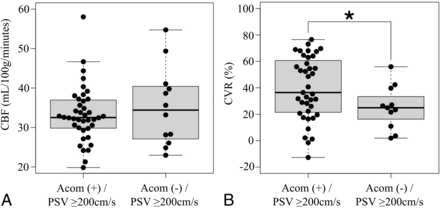
Of the 83 patients, 61 patients were classified into the AcomA (+) group, while 22 patients were classified into the AcomA (−) group. Patients’ baseline characteristics are summarized in Table 1. There were no statistically significant differences in the baseline characteristics between the 2 groups, except in the case of new ischemic lesions on postoperative DWI. Figure 3 shows preoperative ipsilateral CBF and preoperative ipsilateral CVR in response to acetazolamide according to the presence of AcomA flow. Preoperative ipsilateral CBF was not significantly different between the 2 groups (Fig 3A). Although preoperative ipsilateral CVR was higher in the AcomA (+) group than in the AcomA (−) group, the mean difference was not statistically significant (43.4% [SD, 25.3%] versus 31.6% [SD, 22.2%]; P = .056; Fig 3B). In addition, we extracted patients with a PSV ≥200 cm/s, which indicates 70% stenosis,28 from both groups. The patients were subdivided into the AcomA (+)/PSV ≥200 cm/s group (n = 40) and the AcomA (−) / PSV ≥200 cm/s group (n = 12). Preoperative ipsilateral CBF was not significantly different between the 2 groups (Fig 4A). The mean preoperative ipsilateral CVR was significantly lower in the AcomA (−) / PSV ≥200 cm/s group than in the AcomA (+) / PSV ≥200 cm/s group (25.2% [SD, 16.4%] versus 39.6% [SD, 23.8%], respectively; P = .030) (Fig 4B).
Patients’ baseline characteristicsa
A, Box-and-whisker plots of preoperative CBF on the stenotic side in the AcomA (+) group and the AcomA (−) group. Preoperative CBF is not significantly different between the 2 groups. B, Box-and-whisker plots of preoperative CVR on the stenotic side in the AcomA (+) group and the AcomA (−) group. The CVR to the acetazolamide challenge is not significantly different between the 2 groups. The thick horizontal lines divide the boxes at the median values. The bottom and top of the boxes indicate the first and third quartiles. The whiskers extend to the most extreme data points, which are no more than 1.5 times the interquartile range from the box.
A, Box-and-whisker plots of preoperative CBF on the stenotic side in the AcomA (+)/PSV ≥200 cm/s group and the AcomA (−) / PSV ≥200 cm/s group. Preoperative CBF is not significantly different between the 2 groups. B, Box-and-whisker plots of preoperative CVR on the stenotic side in the AcomA (+) / PSV ≥200 cm/s group and the AcomA (−) / PSV ≥200 cm/s group. The CVR to the acetazolamide challenge is significantly lower in the AcomA (−) / PSV ≥200 cm/s group than in the AcomA (+) / PSV ≥200 cm/s group. The thick horizontal lines divide the boxes at the median values. The bottom and top of the boxes indicate the first and third quartiles. The whiskers extend to the most extreme data points, which are no more than 1.5 times the interquartile range from the box. The asterisk indicates P < . 05.
Association between AcomA Flow and the Change in rSO2 before-versus-after Temporary ICA Occlusion
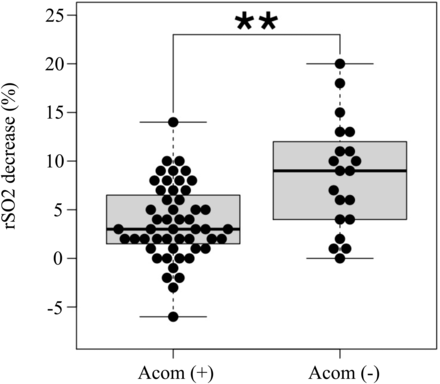
Of 83 patients, 80 patients underwent carotid revascularization. Although CAS was attempted in 2 patients, the guiding catheter failed to navigate into the common carotid artery and the procedure was abandoned. One patient refused CEA immediately before the procedure. CEA was performed in 48 patients, and CAS was performed in 32 patients. The mean decrease in rSO2 after ICA occlusion during CEA or CAS was significantly greater in the AcomA (−) group than in the AcomA (+) group (8.5% [SD, 5.6%] versus 3.7% [SD, 3.8%], respectively; P = .002) (Fig 5).
Box-and-whisker plots of the decrease in rSO2 after temporary ICA occlusion on the stenotic side during CEA or CAS. The decrease in rSO2 is significantly greater in the AcomA (−) group than in the AcomA (+) group. The thick horizontal lines divide the boxes at the median values. The bottom and top of the boxes indicate the first and third quartiles. The whiskers extend to the most extreme data points, which are no more than 1.5 times the interquartile range from the box. Double asterisks indicate P < . 01.
Association between AcomA Flow and New Ischemic Lesions on MR Imaging
New ischemic lesions in the ICA territory ipsilateral to the surgery site on postoperative DWI were recognized in 23 patients (DWI-positive group), while there were no new lesions in 57 patients (DWI-negative group). In the 23 patients with new ischemic lesions, 2 were symptomatic, while 21 were asymptomatic. Among 48 patients who underwent CEA, 7 patients had CEA with carotid shunting, while 41 had CEA without carotid shunting. There were no statistically significant differences in the incidence of new ischemic lesions on postoperative DWI between the patients who underwent CEA with carotid shunting (3/7 [43%]) or without carotid shunting (5/41 [12%]) (P = .080).
In the univariate analysis of factors related to postoperative ischemic lesions on MR imaging, there were significant differences between the DWI-positive group and the DWI-negative group in relation to age, the percentage of subjects undergoing CAS, and the percentage of subjects with AcomA flow (Table 2). The mean age of patients was 78.0 (SD, 5.7) years in the DWI-positive group and 74.4 (SD, 7.3) years in the DWI-negative group (P = .023). The percentage of subjects undergoing CAS was higher in the DWI-positive group than in the DWI-negative group (65% versus 30%, P = .005). The presence of AcomA was more common in the DWI-negative group than in the DWI-positive group (81% versus 52%, P = .014). There were no statistically significant differences between the 2 groups in the preoperative CVR and the rate of ulcerated plaques.
Univariate and multivariate logistic regression analyses of factors for new ischemic lesions on MR imaginga
In the multivariate analysis, the presence of AcomA (OR, 0.07; 95% CI, 0.012–0.45; P = .005) was significantly associated with a decreased odds of new ischemic lesions on postoperative DWI. CAS (OR, 12.99; 95% CI, 2.09–80.86; P = .006) and age (OR, 1.14; 95% CI, 1.01–1.29; P = .039, estimated odds of outcome for a 1 increase in age) were significantly associated with an increased odds of new ischemic lesions on postoperative DWI (Table 2).
The results of univariate analysis of factors related to postoperative ischemic lesions on MR imaging in patients with a PSV ≥200 cm/s are summarized in Table 3. The mean age of patients was 78.7 (SD, 4.3) years in the DWI-positive group and 75.0 (SD, 7.4) years in the DWI-negative group (P = .032). The presence of the AcomA was more common in the DWI-negative group than in the DWI-positive group (87% versus 46%, P = .005). Multivariate analysis in patients with a PSV ≥200 cm/s revealed that only the presence of the AcomA (OR, 0.08; 95% CI, 0.014–0.64; P = .017) was significantly associated with a decreased odds of new ischemic lesions on postoperative DWI.
Univariate and multivariate logistic regression analyses of factors for new ischemic lesions on MR imaging in patients with a PSV of ≥200 cm/sa
DISCUSSION
In this study, we analyzed the impact of AcomA flow on perioperative hemodynamic status and new ischemic lesions on MR imaging after carotid revascularization in patients with carotid stenosis. We showed that preoperative ipsilateral CVR was significantly lower in the AcomA (−) group than in the AcomA (+) group when the PSV was ≥200 cm/s. The decrease in rSO2 after temporary ICA occlusion was significantly greater in the AcomA (−) group than in the AcomA (+) group. Furthermore, the multivariate logistic regression analysis demonstrated that CAS, age, and the absence of AcomA flow were significantly associated with new ischemic lesions on MR imaging after carotid revascularization.
The results showed that preoperative ipsilateral CVR was significantly lower in the AcomA (−) group than in the AcomA (+) group in patients with severe cervical ICA stenosis. This result suggested the importance of collateral flow via the AcomA for preservation of CVR in patients with severe cervical ICA stenosis, which is consistent with a previous study.11 In contrast, it might not be necessary for collateral flow via the circle of Willis to develop for preservation of CVR in mild cervical ICA stenosis; the possibility is supported by no significant difference in CVR between the AcomA (−) group and AcomA (+) group in all patients in the current study.
The decrease in rSO2 after temporary occlusion of the ICA was significantly greater in the AcomA (−) group than in the AcomA (+) group. These results are consistent with those in a previous study14 and suggest that inadequate collateral flow via the circle of Willis due to the absence of AcomA flow might lead to hypoperfusion during clamping of the ICA.
The absence of AcomA flow was significantly associated with new ischemic lesions on MR imaging after carotid revascularization. Dislodgement of thrombotic material or atherosclerotic debris can lead to new ischemic lesions on MR imaging.23 In addition, decreased blood flow during temporary ICA occlusion impedes the clearance of emboli and can lead to new ischemic lesions on MR imaging.29 During temporary ICA occlusion, collateral flow via the circle of Willis develops to maintain CBF.30 Therefore, inadequate collateral flow in patients without AcomA flow might contribute to new ischemic lesions on MR imaging due to impaired emboli clearance. Our decrease in rSO2 after temporary ICA occlusion supports this hypothesis.
Previous studies have reported that reduced CVR is associated with new ischemic lesions on MR imaging after CEA and CAS.7,8 In these studies, it has been hypothesized that the CVR measured with acetazolamide challenge can identify low MCA blood flow during ICA clamping, which might impair clearance of microemboli, leading to ischemic lesions on MR imaging. However, the CVR measured with the acetazolamide challenge was not associated with ischemic lesions on MR imaging in the present study. Although the reason for the inconsistency between our results and previous studies was unclear, CVR was considered to be affected by not only collateral flows via the AcomA but also the degree of ICA stenosis and collateral flows via the posterior communicating and cortical leptomeningeal arteries. In contrast, our result suggested that collateral flow via the AcomA substantially contributed to MCA blood flow during ICA clamping, which was an important factor for new ischemic lesions on MR imaging. Among patients with reduced CVR, it might be important to recognize the pattern of collateral flow development for predicting new ischemic lesions on postoperative MR imaging.
The occurrence of new ischemic lesions on MR imaging after carotid revascularization has been recently considered to lead to clinical consequences in the long term. New ischemic lesions on DWI are associated with a higher risk of cerebrovascular events.31,32 In addition, the presence of silent ischemic lesions may lead to early-onset cognitive decline and dementia.33 Therefore, it is important to predict the risk of new ischemic lesions on MR imaging after the procedure. The current results suggest that evaluating the presence of AcomA flow using ICA-selective MRA constructed by applying a BeamSAT pulse is helpful for predicting the risk of new ischemic lesions and for deciding the treatment and perioperative management strategies.
There were some limitations to the current study. First, the assessment of AcomA flow by ICA-selective MRA was not quantitative but subjective. However, a previous study demonstrated that interobserver agreement in these measurements was excellent.14 Second, collateral flow via anatomic variants in the posterior circulation might also be important, and such variants were not evaluated in this study. However, previous studies have reported that the collateral route via the AcomA is most important.11,14 Therefore, we focused on the presence of AcomA to establish a simple method for predicting new ischemic lesions on MR imaging after carotid revascularization. Third, collateral flow via the ophthalmic artery and via the cortical leptomeningeal artery could not be assessed using this MR imaging technique. Finally, the small sample size was the main limitation of this study. We did not include other important risk factors, such as plaque vulnerability on MR imaging, as predictors of new ischemic lesions after the procedure to avoid further overfitting. Thus, a large-scale study is needed to clarify our results.
CONCLUSIONS
In this study, the absence of AcomA flow was associated with a reduced CVR in the ipsilateral MCA territory in patients with severe cervical ICA stenosis and with hypoperfusion during temporary ICA occlusion. In addition, the absence of AcomA flow was associated with the occurrence of new ischemic lesions after CEA and CAS. Evaluating AcomA flow on ICA-selective MRA might be helpful for predicting the risk of ischemic lesions after the procedure and determining the treatment and perioperative management strategies for ICA stenosis.
Acknowledgments
The authors would like to thank Katsusuke Kyotani and Youta Takemoto for their technical assistance with the MR imaging study and Kazuhiro Kubo for his technical assistance with the SPECT study.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received February 20, 2022.
- Accepted after revision May 17, 2022.
- © 2022 by American Journal of Neuroradiology