Abstract
BACKGROUND AND PURPOSE: Primary intracranial pure yolk sac tumor is very rare. Our aim was to summarize the characteristics of primary intracranial pure yolk sac tumors from the clinical and imaging aspects in a retrospective study.
MATERIALS AND METHODS: We studied 5 patients with primary intracranial pure yolk sac tumors in Guangzhou Women and Children’s Medical Center from January 2015 to June 2021. A comprehensive literature search was performed on the electronic database of the China National Knowledge Infrastructure (1990 to June 2021). Clinical data based on age, sex, treatment, CT, and MR imaging findings were collected and analyzed.
RESULTS: A total of 25 patients were included in the study, 21 boys and 4 girls. Twenty-one patients underwent plain MR imaging and an enhanced examination, 9 patients underwent DWI, and 12 patients underwent plain CT and/or an enhanced examination. The tumors were posterior fossa in 9 cases and supratentorial in 16 cases. All tumors showed marked enhancement after enhanced scanning by MR imaging or CT. The signal on DWI was similar to that of the cerebral cortex, and the ADC map was similar to or slightly higher than that of the cerebral cortex. Among the cases, 13 were followed up from 2 months to 5 years. There was no recurrence or metastasis in 9 patients with postoperative chemotherapy or chemoradiotherapy followed up for 1.5–5 years. Four patients died 2 months to 1.5 years after only an operation, or chemoradiotherapy but no operation.
CONCLUSIONS: There are some relatively specific imaging findings of primary intracranial yolk sac tumors that could assist in their diagnosis. Surgery combined with radiation therapy and/or chemotherapy can achieve a better prognosis.
ABBREVIATIONS:
- AFP
- α-fetoprotein
- YST
- yolk sac tumor
Yolk sac tumor (YST), also known as endodermal sinus tumor, is a highly malignant tumor originating from primitive germ cells.1 It usually occurs in the gonads (testes, ovaries) but can be found outside the gonads in rare cases. YST can be divided into pure and mixed types.2 Primary intracranial YST is very rare, accounting for 2%–5% of intracranial tumors and 6.5% of intracranial germ cell tumors,3 with a peak onset at 10–12 years.4 Most patients with primary intracranial YST have a poor prognosis, with 1-, 3-, and 5-year survival rates of 65.2%, 47.3%, and 40.5%, respectively.4
Due to the extremely low incidence of primary intracranial pure YSTs, imaging reports are even rarer. At present, there are mainly just some case reports.5⇓-7 In the present study, we retrospectively analyzed 5 cases of pure intracranial YST from our center and reviewed all previously reported intracranial pure YST cases in China. The purpose of our study was to summarize the typical clinical characteristics and imaging appearance of primary, intracranial pure YSTs to better understand their characteristics.
MATERIALS AND METHODS
Clinical Patients
We studied 5 cases of primary, intracranial pure YST in Guangzhou Women and Children’s Medical Center from January 2010 to June 2021 (Online Supplemental Data). Patients were diagnosed by surgical pathology and immunohistochemistry. Blood serum was obtained to measure α-fetoprotein (AFP) levels. This study was approved by the ethics committee of Guangzhou Women and Children’s Medical Center (KY 2020–57900). Informed consent was obtained from all of the parents or legal guardians.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used to collect the data. The terms “primary intracranial yolk sac tumor,” “primary intracranial endodermal sinus tumor,” and “malignant germ cell tumor” were searched using the electronic database of the China National Knowledge Infrastructure (1990 to June 2021). A total of 20 qualified cases were obtained (Online Supplemental Data).
The inclusion criteria of the cases in the literature were as follows: 1) younger than 18 years of age, 2) first onset, 3) primary intracranial, 4) pure yolk sac tumor, and 5) published in journals and magazines. Exclusion criteria were the following: 1) 18 years of age or older, 2) metastatic yolk sac tumor, 3) YST complicated by other components (mixed type), 4) cases with incomplete clinical or imaging data, and 5) the case not Chinese.
Imaging Protocols
Four patients in our center underwent scanning on a Magnetom Skyra 3T scanner (Siemens). In 4 cases, MR imaging–enhanced head scans were performed, and DWI scans were performed in 3 cases. The scans had the following parameters: T1WI: TR/TE = 500∼600 ms/8∼10 ms; T2WI: TR/TE = 3500–4200/100–120 ms; T1WI gradient-echo fat-suppression sequence: TR/TE = 250–500/5–10 ms; DWI: TR/TE = 3500–4200/60–80 ms, b-value = 800 seconds/mm2. All the sequence parameters were as follows: thickness/gap = 5 /1 mm, FOV = 240 × 240 mm, matrix = 320 × 320. The dose of contrast agent was 0.1 mmol/kg.
Two cases in our center underwent head contrast-enhanced CT. The CT scanning protocol consisted of the axial plane using a 64-section spiral CT machine (tube voltage = 120 kV, automatic current conditions, thickness = 1 mm, layer spacing = 1 mm, matrix = 512 × 512, and a standard algorithm reconstruction image with a 5-mm-layer-thickness multiplanar reconstruction and volume rendering). Contrast-enhanced CT was performed following intravenous injection of 2 mL/kg of nonionic contrast medium.
For uncooperative children, chloral hydrate (0.5 mL/kg) was administered orally for sedation, and they were scanned when in quiet sleep.
In the literature,8⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–22 17 patients underwent contrast-enhanced MR imaging, and 6 patients underwent DWI scans. Two patients underwent plain head CT scans, and 8 patients underwent enhanced head CT scans.
Image Analysis
All cases were reviewed by 2 pediatric neuroradiologists, and consensus was reached after discussion in case of disagreement. Information was collected about the lesion location, size, morphology, signal or density, enhancement characteristics, and surrounding edema, mass effect, hydrocephalus, and so forth.
RESULTS
Clinical Data Characteristics
Among the 25 cases, 21 were boys and 4 were girls, from 1 year and 10 months of age to 17 years of age, with a median age of 10 years (Table). Before surgery, the serum AFP was increased in 6 cases, all of which were >1000 ng/mL (normal value = 0–8.1 ng/mL) and the serum AFP gradually decreased to normal after surgery. In a case with tumor recurrence, the AFP increased significantly after surgery. The tumor was resected in 23 cases, and only a biopsy was performed in 2 cases. Thirteen cases were followed up from 2 months to 5 years. Three patients were followed up after surgery combined with radiation and chemotherapy for 1.5 years, 4 years, and 5 years, respectively, without recurrence. One patient was followed up for 2 years after surgical resection without recurrence. Two patients died 2 months after surgery, and 2 patients died 6 months and 1.5 years after radiation therapy and chemotherapy. Four patients were followed up with surgery combined with chemotherapy for 1.5 years, 2 years, 3 years, and 3 years, respectively, without recurrence. One patient did not receive chemotherapy after surgery due to family economic reasons. After 6 months of outpatient follow-up, his blood AFP increased significantly and tumor recurrence was found by MR imaging examination. Surgical treatment was performed again, and standard chemotherapy was performed after surgery. There were no signs of a second recurrence after 4 years of follow-up.
Clinical manifestations of 25 patients with primary, intracranial, pure YST
Imaging Findings
Of the 25 patients with primary intracranial YST, 9 cases were located in the posterior fossa and 16 cases were located in the supratentorial lateral ventricle. In 25 cases, the maximum diameter line was 2.5–7.0 cm, the boundary was relatively clear, and there were different degrees of mass effect with no edema or only mild edema around the tumor. There were 19 cases with different degrees of obstructive hydrocephalus in the supratentorial ventricle and 6 cases without hydrocephalus. Sixteen cases were quasicircular, and 9 cases were irregular. Nine cases were cystic solid with solid components, and 16 cases were solid.
Thirteen cases of tumors were solid, of which 11 cases had relatively uniform signals, with multiple faint microcysts showing slightly low signals on T1WI and slightly high signals on T2WI; 2 cases had uniform signals, with slightly high signals on T1WI and slightly high signals on T2WI. Eight cases were cystic solid lesions (Fig 1), dominated by solid components; cystic components could be seen around or in the center of the tumor. Solid components showed slightly lower signals on T1WI and slightly higher signals on T2WI. After enhanced scanning, the solid components of the solid tumors and cystic solid tumors were significantly enhanced, with multiple, thick, serpiginous enhancement (Fig 1) and multiple unenhanced microscopic cystic components, which were increased compared with plain scanning. The was no enhancement of the cystic components. In 2 cases, the signal was uniform and the tumor enhancement was uniform. Nine DWI signals were equisignal (compared with the cerebral cortex) (Figs 1 and 2), and 7 ADC signals were equisignal or slightly high (compared with the cerebral cortex).
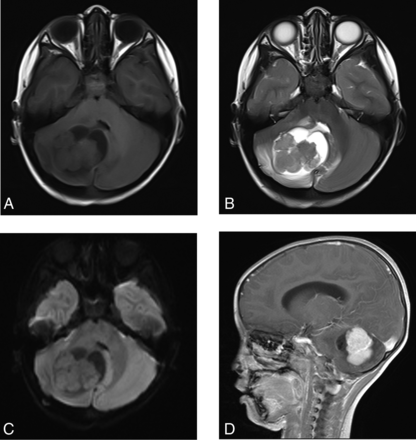
A 4-year-old boy with a right cerebellar hemisphere YST. A, MR image shows the tumor located in the right cerebellar hemisphere. It is solid with cystic changes. B, There is a small amount of edema around the tumor, and the fourth ventricle is compressed. C, DWI shows isosignal of solid components of the mass. D, The tumor shows serpiginous enhancement.
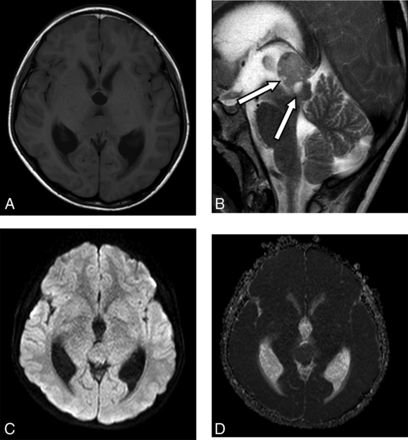
An 11-year-old boy with a pineal region YST. A, MR image shows the tumor located in the pineal region with a slightly low signal associated with supratentorial dilation of the ventricle. B, The T2 sequence shows multiple, fine cysts with hypersignal (arrows). C and D, The DWI sequence and ADC map show that the tumor signal is the same as that of the cerebral cortex.
In 12 patients, 1 patient had slightly low density, 3 patients had isodense, and 8 patients had uniform or slightly high density. Three patients had peritumor or central cystic changes, and 3 patients had peritumor calcification. Ten cases had obvious enhancement, among which 6 cases had irregular enhancement (Fig 3).
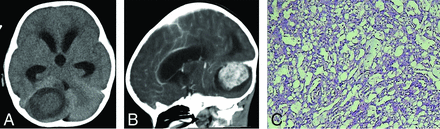
A boy, 2 years and 10 months of age, with a right cerebellar hemisphere YST. A, CT shows a slightly hypodense mass lesion accompanied by peritumoral edema. B, Enhanced CT shows heterogeneous density. C, H&E staining shows a typical Schiller-Duval body and loose, vacuolated network structures and prominent nucleoli with more atypia cells.
Pathologic Features
The gross specimen of the tumor was tough and rich in blood supply. Microscopically, the tumor tissue was distributed in a loose network. The tumor cells were rich in cytoplasm, red-stained or transparent, and the nuclei were large, round, or oval, with obvious atypia and abundant nuclear mitosis. Eosinophilic and Schiller-Duval bodies were seen locally, and hemorrhagic necrosis was seen in the stroma. Immunohistochemical results were as follows: AFP (+), glypican-3 (+), cytokeratin (+), sal-like protein 4 (+), focal (+), Ki67 (20∼70% (+), CD30 (−), octamer-binding transcription factor 4(−).
DISCUSSION
Primary intracranial pure YST is a rare and highly malignant germ cell tumor that is usually located in the pineal and the saddle regions. Primary YST occurring in the posterior fossa is even rarer.23 In this study, the incidence of primary intracranial YST was significantly higher in boys (21/25, 84%), which was slightly higher than that in a report about the prevalence of YST in children and adolescents (males 132/191, 69%).2 However, this report covered YST in multiple systemic systems and did not discuss the incidence of intracranial YST separately. To date, no more than 20 cases of posterior fossa YST have been reported in PubMed,23,24 all of which were male and mostly in Asian races, mainly in Japan, China, and South Korea. In this study, tumors in 9 patients (9/25, 36%), all of whom were male, were located in the posterior cranial fossa. The high proportion of tumors occurring in the posterior cranial fossa may also be related to the sample size collected in this study. To date, primary intracranial pure YST in the posterior fossa has not been reported in any girls worldwide, possibly indicating that it only occurs in boys, but more cases need to be confirmed in the future.
Due to the low incidence and high degree of malignancy of intracranial YST, there are no relevant treatment guidelines at present. However, recent studies suggest that surgical resection followed by chemoradiotherapy and/or chemotherapy can improve the survival rate of patients with intracranial, primary YST.23,25,26 In this study, 13 patients were followed up, and there was no recurrence or metastasis in patients with surgery combined with chemoradiotherapy or surgery combined with chemotherapy for 1.5–5 years (8/13, 62%), while patients with surgery alone or chemoradiotherapy alone died after 2 months to 1.5 years (4/13, 3%). This finding suggests that surgical resection combined with standard chemotherapy or radiation therapy and chemotherapy can improve the survival rate of children with primary, intracranial, pure YST, a finding consistent with that in previous literature reports.
Because YST originates from the endoderm, it retains the ability to synthesize AFP in the fetus, and AFP levels are high in most patients. Therefore, elevated blood AFP levels are considered a sensitive indicator for the diagnosis of YST.27 In this study, the blood AFP of 6 children was significantly increased before surgery and decreased to the normal level after surgery, and in 1 case, AFP was also significantly increased after recurrence. Therefore, we believe that the serum AFP level should be checked regularly after surgery to detect recurrences early.
The imaging findings of intracranial primary YST are considered nonspecific,28⇓-30 but the imaging findings of this group of cases have certain characteristics. Intracranial primary YST presented as a round mass (16/25, 64%) with a clear boundary and an obvious mass effect, but the surrounding edema was usually mild and CT showed slightly higher, equal, or slightly lower density. On MR imaging, the solid components of intracranial primary YST showed equal or slightly lower T1WI signals and slightly higher T2WI signals, and a small part showed slightly higher T1WI signals, which may be related to the presence of mucous components in the tumor or the rich capillary and sinusoid structures in the tumor. In this study, 21 cases of MR imaging and 6 cases of CT were significantly enhanced with uneven enhancement, in which there were many microcystic or cystic components and many large, twisted blood vessels in the solid enhancement components, suggesting that the tumor was rich in blood supply, which was consistent with the pathologic features reported in the literature.16
Four cases had uniform enhancement after CT enhancement, which may be because the soft-tissue resolution of CT is lower than that of MR imaging, and tiny cystic components in the tumor were not visible. Combined with the cases in this study and relevant literature,28,31,32 we believe that cystic changes, especially multiple microvesicles and multiple thickening and twisting tumor vessels, are one of the imaging features of primary intracranial pure YST, corresponding to pathologic changes.
DWI shows the disorderly diffusion movement of free water by calculating the ADC value, which is manifest as a high signal on DWI and a low ADC value, reflecting the size of the extracellular space, number of cells, and the ratio of cytoplasm to extracellular space.33 In most malignant tumors,34⇓-36 due to the dense distribution of tumor cells, large nuclei, small extracellular intervals, and limited diffusion of free water movement, DWI showed a high signal and a reduced ADC value, suggesting limited diffusion. Similar to previous reports,28,32 9 cases in this study had equal signals on DWI and equal or slightly higher signals on ADC, suggesting that diffusion was not limited. It is speculated that this finding may be related to the loose network structure of tumor tissue and the large extracellular space. Therefore, we believe that DWI can be used to differentiate most malignancies, such as lymphoma, medulloblastoma, and atypical teratoma/rhabdomyoid tumor.
YST in the pineal region is mainly differentiated from pineal tumors, germ cell tumors, and non-germ cell tumors. YST in the cerebellum is mainly differentiated from medulloblastoma, astrocytoma, and atypical teratoma/rhabdomyoid tumor. Combined with laboratory AFP levels are helpful in the diagnosis of YST. In addition, although AFP is a characteristic marker of YST, it is not specific and can also be expressed in other germ cell–derived tumors and many non-germ cell tumors. It is still difficult to distinguish intracranial YST from other mixed germ cell tumors with YST components on imaging.
The limitation of this study is its retrospective analysis. Some cases are from the literature, and the clinical and imaging data are limited, a feature that needs further improvement. In addition, we studied only cases of Chinese children, so there may be some biases for non-Chinese cases.
CONCLUSIONS
Primary intracranial pure YST is extremely rare and is mostly seen in boys. The imaging findings of this disease have certain characteristics, such as multiple microsacs in the tumor; significant enhancement, and multiple thick, serpiginous enhancement; equal signal on DWI; and equal or slightly higher signal on ADC combined with the serum AFP level, which is helpful for the diagnosis of this disease, but the diagnosis depends on pathologic and immunohistochemical examinations.
Footnotes
This work was supported by the General Guidance Project of Guangzhou Health Science and Technology Project (20211A010021).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received February 4, 2022.
- Accepted after revision May 4, 2022.
- © 2022 by American Journal of Neuroradiology