Abstract
BACKGROUND AND PURPOSE: The use of gadolinium-based contrast agents contributes to the cost of MR imaging and prolongs image-acquisition time. There are also recent concerns regarding gadolinium deposition, particularly in patients who require frequent follow-up MRIs. The purpose of this study was to assess whether gadolinium-based contrast agents are needed during MR imaging follow-up for unoperated pituitary macroadenoma.
MATERIALS AND METHODS: A total of 105 patients with unoperated pituitary macroadenoma who underwent follow-up MR imaging of the sella were included in this retrospective study. The craniocaudal dimension, cavernous sinus invasion grading, and optic pathway compression were assessed independently on coronal T2WI and compared with coronal T1-weighted images with gadolinium-based contrast agents (T1 postcontrast images). The agreement between the T2WI and T1 postcontrast images for the craniocaudal dimension was assessed using the intraclass correlation coefficient; for the cavernous sinus invasion and optic pathway compression, it was assessed using κ statistics.
RESULTS: There was excellent agreement for the craniocaudal dimensions between T2WI and T1 postcontrast images (intraclass correlation coefficient = 0.96, P < .001; 95% CI, 0.84–0.99). Additionally, there was almost-perfect agreement between cavernous sinus invasion and optic pathway compression between T2WI and T1 postcontrast images, with κ = 0.95 and 0.84, respectively (P < .001).
CONCLUSIONS: MR imaging of the sella without the use of gadolinium-based contrast agents could potentially be considered for the follow–up of unoperated pituitary macroadenomas. This choice can reduce the MR imaging examination cost and acquisition time and avoids potential adverse effects of gadolinium-based contrast agents.
ABBREVIATIONS:
- GBCA
- gadolinium-based contrast agent
- ICC
- intraclass correlation coefficient
- T1C
- T1 postcontrast images
Pituitary adenoma is a benign slow-growing tumor with an estimated prevalence of 14.4% in postmortem and 22.5% in imaging studies.1 Macroadenomas represent up to 31% of pituitary adenomas in epidemiologic studies.2,3 Patients with macroadenoma who are not candidates for upfront surgery or elect not to have surgery require MR imaging follow-up to assess tumor size, cavernous sinus invasion, and mass effect on the optic pathways with commonly used gadolinium-based contrast agents (GBCAs).
GBCAs increase the cost of MR imaging, prolong scan time, and require insertion and discontinuation of intravenous access, which further contribute to scan costs. GBCAs are also associated with adverse reactions at a rate of 0.07%–2.4%.4,5 Additionally, repeat GBCA administration can lead to permanent gadolinium deposition in the brain.6⇓⇓⇓-10 The clinical significance of these deposits is unknown. For these reasons, the risks must be weighed against benefits before administering GBCA.
The purpose of this study was to assess whether GBCAs are needed for routine MR imaging follow-up of unoperated pituitary macroadenomas. We hypothesized that coronal T2WI and coronal post-GBCA T1-weighted images (T1C) have the same performance for assessment of craniocaudal dimension, cavernous sinus invasion, and optic pathway compression in patients with unoperated pituitary macroadenomas.
MATERIALS AND METHODS
This retrospective, single-center study was approved by local institutional review board (King Abdullah International Medical Research Center) with a waiver of informed consent. The initial search of our data base including patients 14 years of age and older with a diagnosis of pituitary macroadenoma who underwent MR imaging of the sella between January 2010 and September 2021 revealed 119 consecutive patients. Five were excluded because they did not have GBCA-enhanced scans, and 9 were excluded because they had prior pituitary surgery. A total of 105 patients were included in the study (Fig 1).
Flow chart shows patient selection.
MR Imaging Scanning Settings
The patients were scanned on 3T Achieva (Philips Healthcare), 1.5T Espree (Siemens), or 3T Discovery (GE Healthcare) scanners. The imaging parameters for coronal T1C and coronal T2WI are detailed in Table 1. Gadoterate meglumine (Dotarem; Guerbet) was used as the GBCA for all patients.
MR imaging parameters
Image Analysis
The first routine MR imaging follow-up of the sella was used for analysis to ensure that the dedicated pituitary protocol was performed for all patients. All scans were anonymized, and we constructed 2 separate batches: One contained only coronal T2WIs, and the other one contained only coronal T1Cs. To avoid potential bias, we analyzed the T1C batch 1 month after the T2WI batch analysis was completed. Analysis was performed by a neuroradiologist with 4 years of experience (A.A.A., reader 1), who was blind to all clinical data.
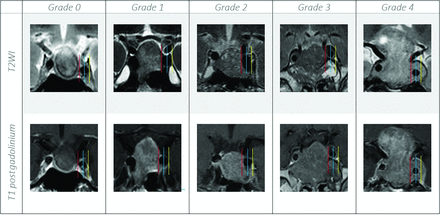
For each case, the maximum craniocaudal dimension of the macroadenoma was measured. The cavernous sinus invasion was assessed using the Knosp classification (Fig 2).11 The optic pathway (prechiasmatic optic nerves, optic chiasm, or optic tracts) compression was assessed and classified into 3 categories: no contact, abutment without displacement, and compression with displacement.
Coronal T2WI versus T1C show the different grades of cavernous sinus invasion using the Knosp classification; grade 0, the lesion does not extend beyond the medial carotid line (red line); grade 1, the lesion extends to the medial line but does not reach the intercarotid line (blue line); grade 2, the tumor extends beyond the intercarotid line but does not extend beyond the lateral line (yellow line); grade 3, the tumor extends to the lateral line more so on the left (yellow line); and grade 4, there is complete encasement of the cavernous carotid artery.
To assess interrater reliability, we randomly chose 31 patients, and another neuroradiologist with 10 years of experience (P.B.H., reader 2) reviewed them independently using the same method as reader 1.
Statistical Analysis
Descriptive statistics were used for continuous variables including mean (SD) and range. A difference of ≥2 mm along the craniocaudal dimension between the baseline and follow-up examination was considered notable growth or shrinkage. The agreement between the coronal T2WI and T1C for the craniocaudal dimension was assessed using the intraclass correlation coefficient (ICC).12 The agreement of the cavernous sinus invasion and optic pathway compression was assessed using κ statistics.13
All P values were 2-sided, and a P < .05 was considered statistically significant. Statistical analysis was performed using STATA, Version 17 (StataCorp).
RESULTS
A total of 105 patients, including 57 women (54.28%) and 48 men (45.71%), with pituitary macroadenoma were included in this study, with a mean age of 48.5 (SD,15.79) years (range, 16–87 years).
Analysis of Interval Growth
The mean time between the baseline and analyzed scans was 10.41 (SD, 5.96) months (range, 2–36 months). Thirty-eight/105 patients had routine brain MR imaging without dedicated sellar sequences as their baseline scan. To assess interval growth, we excluded these, with the remaining 67 patients with dedicated MR imaging of the sella for both the baseline scans and the scans included in this study.
Thirty-four/67 (50.74%) patients were stable in their craniocaudal dimension: 16/67 (23.88%) increased and 17/67 (25.37%) decreased at almost-perfect agreement between the T2WI and T1C with a κ of 0.9 (P < .001). Analysis of the interval change revealed no significant difference in the craniocaudal dimensions between the T2WI and T1C (P = .85) and also excellent agreement between T2WI and T1C (ICC = 0.98, P < .001; 95% CI, 0.96–0.98).
By means of T2WI, the average growth or regression of the craniocaudal dimension from baseline to the follow-up scan was 3.8 (SD, 2.1) mm (range, 2–8 mm) and 6 (SD, 4.3) mm (range, 2–14 mm), respectively.
Analysis of the Craniocaudal Dimension
The mean craniocaudal dimension of macroadenomas on T2WI was 23.13 (SD, 10.73) mm (range, 9–55 mm) compared with 23.14 (SD, 10.79) mm (range, 8–55 mm) on the T1C (P = .99) (ICC = 0.96; P < .001; 95% CI, 0.84–0.99) (Table 2).
Summary statistics comparing the craniocaudal dimension of pituitary macroadenoma, cavernous sinus invasion, and optic pathway compression on T2WI versus T1C
Analysis of the Cavernous Sinus Invasion
There were 16 patients classified as Knosp zero, 28 as Knosp one, 33 as Knosp two, 15 as Knosp 3, and 13 as Knosp 4, with almost-perfect agreement between the T2WI and T1C with κ = 0.95 (P < .001).
Analysis of the Optic Pathway Compression
The macroadenoma showed no contact with the optic pathway in 32 (30.47%) patients, while there was abutment of the optic pathway without displacement in 18 (17.14%) and mass effect and displacement in 55 (52.38%) patients (Fig 3). There was almost perfect agreement between the T2WI and T1C regarding the optic pathway compression with κ = 0.84 (P < .001).
Coronal T2WI versus T1C show the optic pathway (depicted by yellow arrows) compression classification. A, There is no contact. B, The pituitary macroadenoma is abutting the left aspect of the optic chiasm without displacement. C, There are mass effect and displacement of the optic pathway.
Subgroup Analysis of Macroadenoma with Internal Heterogeneity
A total of 47 (44.76%) of 105 patients demonstrated a heterogeneous appearance on the T2WI sequence. Subgroup analysis of the heterogeneous macroadenomas revealed no significant difference in the craniocaudal dimensions (P = .98), but it did have excellent agreement between the sequences, (ICC = 0.99, P < .001; 95% CI 0.998–0.999). Analysis of the heterogeneous macroadenomas revealed less but still almost-perfect agreement between T2WI and T1C for cavernous sinus invasion with κ = 0.94 (P < .001) and substantial agreement for optic pathway compression with κ = 0.74 (P < .001).
Interrater Reliability
There were 31 patients randomly chosen and reviewed independently by both reader 1 and reader 2 for the craniocaudal dimensions, cavernous sinus invasions, and optic pathway compression using coronal T2WI. Analysis of this subset revealed excellent interrater reliability between the 2 readers for craniocaudal dimensions (ICC = 0.95, P = .001; 95% CI, 0.61–0.99) and substantial agreement for cavernous sinus invasion with κ = 0.76 (P < .001). There was also substantial agreement for the optic pathway compression with κ = 0.77 (P < .001).
DISCUSSION
In this study, we demonstrated that coronal T2WI and T1C perform similarly for assessment of craniocaudal length, cavernous sinus invasion, and optic pathway compression in patients with unoperated pituitary macroadenomas. MR imaging follow-up in this group of patients without the use of GBCA can provide safety improvement for patients and operational cost-savings.
Surgery is the standard of care for pituitary macroadenomas, particularly if symptomatic and large. However, up to 30% of pituitary macroadenomas are managed conservatively instead of with surgery for several reasons:14 Some patients are not surgical candidates due to comorbidities. The benefits of upfront surgery for nonfunctioning asymptomatic pituitary macroadenoma or prolactin-secreting macroadenoma without optic pathway compression for patients undergoing medical treatment remains questionable.15 Local practice patterns may also affect the decision to pursue upfront surgery rather than imaging follow-up in patients with pituitary macroadenoma. In addition, up to 24% of macroadenomas demonstrate evidence of growth with time if untreated.16 Therefore, there is a subgroup of patients with pituitary macroadenoma that do not undergo upfront surgery but need serial imaging follow-up annually or, in those patients in whom there is a high demonstrated growth rate, even more frequently.17,18
On the basis of our results, administration of GBCA has no added value to the follow-up MR imaging examinations for unoperated pituitary macroadenomas to assess tumor size. We assessed cavernous sinus invasion because it affects the surgical approach, risks, and outcomes.19 Cavernous sinus invasion is associated with increased risk of residual disease and the need for future interventions20 following surgery. This knowledge is necessary for risk-benefit assessment in patients with macroadenoma who are on conservative imaging surveillance. Visual pathway compression is also significant because the presence of visual impairment is a potential indication for surgery,21 affecting surgical outcomes.22,23 In addition, our results demonstrated no added value of performing coronal T1C compared with coronal T2WI in the assessment of heterogeneous-versus-nonheterogeneous macroadenomas.
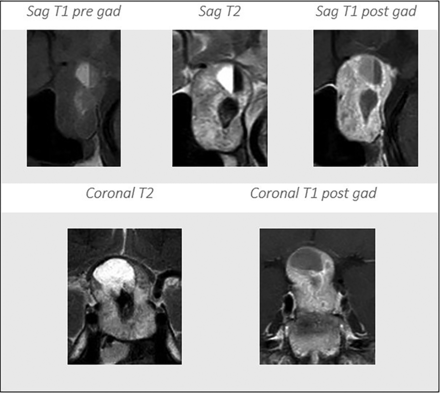
Multiple prior studies have also demonstrated a lack of added clinical value of using GBCA for routine follow–up scans of benign intracranial tumors such as meningiomas24 and vestibular schwannomas.25 The contrast-enhanced scans are associated with increased cost, including the GBCAs themselves, longer scan times, and additional support staff for IV access insertion and removal. They are also associated with more patient discomfort and the potential for adverse reactions to GBCA. In addition, repeat GBCA administrations lead to permanent deposition of gadolinium in the brain, and, in particular, in the dentate nucleus and globus pallidus.6⇓⇓⇓-10 Although the current study was designed to compare T2WI and T1C, in practice the non-contrast-enhanced MR imaging of the sella includes T1-weighted images, which provide more information with regard to internal hemorrhage and the presence of fluid-fluid levels, a posterior pituitary bright spot, and bone marrow in the clivus compared with T2WI alone (Fig 4).
MR imaging of the sella shows internal hemorrhage with the hematocrit level on different sequences. Sag indicates sagittal; pre gad, pregadolinium; post gad, postgadolinium.
Limitations
The retrospective single-center nature of this study and limited sample size are sources of bias. Volumetric assessment of pituitary macroadenoma is the most accurate measure of its size. However, this is not applicable to daily clinical practice, and we, therefore, assessed the craniocaudal dimension due to its importance to optic pathway compression. Last, because local practice patterns can affect whether a patient with macroadenoma will undergo upfront surgery, different practices may have different numbers of patients to whom the results of this study could be applicable.
CONCLUSIONS
Our study suggests that non-contrast-enhanced MR imaging of the sella could potentially be considered for the follow–up scans of unoperated pituitary macroadenomas. The results need to be validated in larger, multicenter studies.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received March 28, 2022.
- Accepted after revision May 4, 2022.
- © 2022 by American Journal of Neuroradiology