Abstract
BACKGROUND AND PURPOSE: Considerable overlap exists in the MR imaging features of hypoglycemic injury and hypoxic-ischemic brain injury, with similar predilections for the occipital and parietal lobes. In partial, prolonged hypoxia-ischemia, there is cortical destruction at the interarterial watershed zones, and in concomitant hypoglycemia and hypoxia-ischemia, an exaggerated final common pathway injury occurs. We interrogated secondary white matter tract–based thalamic injury as a tool to separate pure injuries in each group.
MATERIALS AND METHODS: A retrospective observational study of the MRIs of 320 children with a history of hypoxia-ischemia and/or hypoglycemia was undertaken with 3 major subgroups: 1) watershed-type hypoxic-ischemic injury, 2) neonatal hypoglycemia, and 3) both perinatal hypoxia-ischemia and proved hypoglycemia. Cerebral and thalamic injuries were assessed, particularly hyperintensity of the posterolateral margin of the thalami. A modified Poisson regression model was used to assess factors associated with such thalamic injury.
RESULTS: Parieto-occipital injuries occurred commonly in patients with hypoglycemia and/or hypoxia-ischemia. Eighty-five of 99 (86%) patients with partial, prolonged hypoxia-ischemia exhibited the thalamus L-sign. This sign was also observed in patients who had both hypoglycemia and hypoxia-ischemia, predominantly attributable to the latter. Notably, the risk of a thalamus L-sign injury was 2.79 times higher when both the parietal and occipital lobes were injured compared with when they were not involved (95% CI, 1.25–6.23; P = .012). The thalamus L-sign was not depicted in patients with pure hypoglycemia.
CONCLUSIONS: We propose the thalamus L-sign as a biomarker of partial, prolonged hypoxia-ischemia, which is exaggerated in combined hypoglycemic/hypoxic-ischemic injury.
ABBREVIATIONS:
- HGI
- hypoglycemic injury
- HIBI
- hypoxic-ischemic brain injury
The MR imaging features of hypoglycemic injury (HGI) and hypoxic-ischemic brain injury (HIBI) are well-documented. In pure HGI, without HIBI, some authors have demonstrated a posterior-predominant pattern of cerebral injury with a predilection for the occipital and parietal lobes.1⇓⇓-4 Other studies have noted that the pattern of HGI may be more widespread and not always limited to the parieto-occipital areas.5 In the partial, prolonged type of HIBI, destruction of the cortex typically involves the interarterial anterior, posterior, and peri-Sylvian watershed zones and the contiguous white matter.6⇓⇓⇓-10 HIBI-associated thalamic injury has been less frequently described, and in this study, we attempted to investigate thalamic involvement in children with documented partial, prolonged HIBI, neonatal hypoglycemia, or combined hypoxic-ischemic and hypoglycemic injuries.
MATERIALS AND METHODS
MR imaging studies performed on 320 term neonates with suspected HIBI and/or HGI were analyzed for specific anatomic patterns of injury. The retrospective, multicenter nature of the study and the various clinical setups did not allow time standardization of imaging; all imaging occurred after the acute phase of injury. Imaging studies were conducted on 1.5T MR imaging scanners (Siemens). The sequences performed in all patients included sagittal T1-weighted volumetric: 1-mm-slice GE (TR/TE = 1900/2.95 ms), coronal volumetric inversion recovery: 1.1-mm-slice spin-echo (TR/TE = 4000/363 ms), axial T2-weighted, axial FLAIR, axial diffusion-weighted/ADC, coronal inversion recovery through the temporal lobes, axial susceptibility-weighted and coronal T2-weighted sequences were obtained in all patients. Ethics approval was obtained from University of KwaZulu-Natal (BREC00001036/2020).
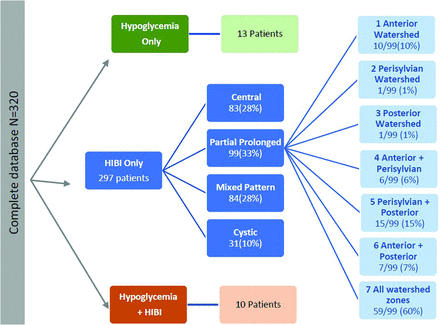
After anonymization, images were reviewed by 2 radiologists (S.K.M. and J.W.L. with 15 and 30 years of experience in neuroradiology respectively), who were blinded to all patient data. Brain MR imaging findings were divided by consensus into 3 major study subgroups (Fig 1). The HIBI subgroup was categorized into 4 patterns; additionally, the partial prolonged HIBI group was further subdivided into 7 (Fig 1) watershed injury pattern subtypes. We noted 3 categories of thalamic involvement: posterolateral/pulvinar, atypical, and no thalamic injury. In particular, the first category referred to those patients with an injured pulvinar and the lateral margin of the thalamus abutting the posterior limb of the internal capsule. In patients with a documented central (basal ganglia–thalamus) pattern or mixed-type HIBI (with partial, prolonged, and central patterns), there is often ventral thalamic injury, usually of the ventral posterior lateral nuclei.6,9 In addition, assessment of the posterolateral aspect of the thalami is difficult in patients who have had multilobar cystic encephalomalacia with severe or total brain injury. Patients with these 3 patterns (central, mixed, and cystic encephalomalacia) of HIBI were excluded, as well as those with incomplete clinical information and poor-quality images.
Derivation of the 3 major study groups, the subgroups of HIBI, and the subtypes of watershed patterns of injury in patients who had partial, prolonged HIBI.
A separate cohort of 13 patients (uppermost in Fig 1) with documented neonatal hypoglycemia was evaluated for thalamic and cerebral injuries, to assess pulvinar and cortical, especially parieto-occipital, injury. Each of these 13 patients had a similar set of MR imaging sequences (as above). These studies were independently reviewed by S.K.M. and J.L.
Blood glucose levels were documented in all neonates who had symptomatic hypoglycemia. Neonatal hypoglycemia was defined by a recorded plasma glucose value of <1.8 mmol/L during the first 2 hours of life or <2.6 mmol/L thereafter.11 Hypoxic-ischemic encephalopathy was excluded on the basis of the absence of fetal distress, normal findings on blood gas analysis, reassuring Apgar scores at 1 and 5 minutes, and the absence of multiorgan hypoxia.
The third group of 10 patients (lowermost in Fig 1) included those in whom both perinatal hypoxic-ischemic encephalopathy and hypoglycemia were documented. Particular attention was paid to identification of injury to the watershed zones and pulvinar involvement in these patients. The Apgar scores together with documented perinatal hypoxia-ischemia and blood gas analyses confirmed HIE, and there was also recorded hypoglycemia in all these children, either after birth or in the first 2 days of life as per the criteria outlined above.
Statistical Analysis
Categoric variables of the key features of each partial, prolonged HIBI subtype and thalamic injury location were expressed as frequencies and percentages and compared using either the χ2 test or the Fisher exact test if there were <5 observations in any cell. A modified Poisson regression model was used to assess factors associated with pulvinar thalamic injury versus nil and atypical subtypes combined. A 2-tailed P < .05 indicated statistical significance. All statistical analyses were conducted using SAS, Version 9.4 (SAS Institute).
RESULTS
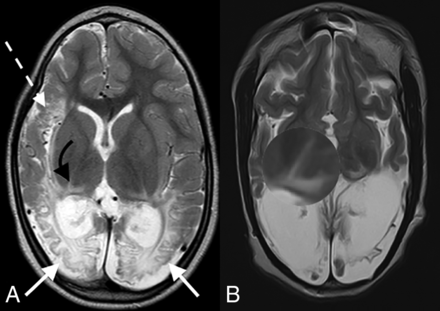
The group of 99 term neonates with HIBI demonstrating partial, prolonged patterns of injury were imaged in the chronic phase of injury, with the average age at the time of imaging being 6 years. This sample (n = 99) included 41 female patients and 58 male patients and were categorized into 7 subtypes, as shown in Fig 1. Table 1 highlights the prevalence, type, and severity of thalamic injury in children with each subtype of partial, prolonged HIBI in relation to the cortical injuries located in the frontal, parietal, occipital, peri-Sylvian, and hindbrain regions. There was a high degree of correlation of the MR imaging features between both readers with no major discrepant findings. Lobar involvement was shown to be high across all 4 cerebral lobes in descending order: occipital (91.9%), parietal (89.9%), frontal (88.9%), and temporal lobes (84.8%) (Table 1). In the subgroup of patients who demonstrated all 3 watershed zone injuries (9/99), we found injury to the posterolateral margin of the thalamus (adjacent to the posterior limb of internal capsule) and posterior thalamic (pulvinar and lateral geniculate) nuclei on both sides, shown in Fig 2, which we term the “thalamus L-sign.” Overall, the thalamus L-sign was present in 86% (85/99) of patients with prolonged, partial HIBI. Furthermore, subtype 7 with involvement of all 3 watershed zones was the most prevalent HIBI subtype among the 85 (69.4%) patients demonstrating the thalamus L-sign injury.
Axial T2-weighted images in a child with partial, prolonged HIBI demonstrating interarterial injuries at the peri-Sylvian (dashed white arrow) and posterior parieto-occipital (solid white arrows) watershed regions. Note the thalamus L-sign (curved arrows in A and highlighted by the loupe in a second patient in B).
Key features of the 7 subtypes of partial prolonged/watershed HIBI
None of the patients (n = 10) with isolated anterior watershed injury had a thalamus L-sign. In some cases, signal abnormalities were evident in the anterior thalamus, usually medially and ventrally, but these never included the ventral posterior lateral nucleus. All patients (61/99) in subtypes 2, 3, and 7 showed a bilateral thalamus L-sign. When the anterior and peri-Sylvian watershed zones were involved together (in subtype 4), we noted that half of those patients had a positive thalamus L-sign, but in each of these, the sign was identified unilaterally, only on the side where the peri-Sylvian cortex was destroyed. One patient in subtype 5 had a feint unilateral posterior thalamic hyperintensity on the side where the watershed cortex destruction was more pronounced, but this was not categorized as a thalamus L-sign. The rest of the patients in subtype 5 all demonstrated a bilateral thalamus L-sign. One patient of subtype 6 did not demonstrate the sign; however, the anterior watershed zone was severely destroyed in this instance, with only minimal posterior watershed involvement. This patient's pattern of injury simulates that of subtype 1.
A key correlation is the concomitant injury at the posterolateral thalamus and the lobes involved in the watershed zones of the cerebrum. There were statistically significant differences between the thalamus L-sign injury versus the other types of injuries combined; in particular, injuries involving the parietal, occipital, and temporal lobes were significantly more prevalent in the thalamus L-sign injury compared with other thalamic injuries (nil and atypical) (Table 2). Critically, the risk of experiencing a thalamus L-sign–type injury was 7.38 times higher when an occipital lobe injury was identified compared with when it was not involved (95% CI, 1.18–46.23), and this finding was statistically significant (P = .033). Similarly, the risk of experiencing a thalamus L-sign injury was 3.07 times higher when a parietal injury was present compared with when it was not involved (95% CI, 1.19−7.93), and this finding was statistically significant (P = .020). Notably, the risk of experiencing a thalamus L-sign injury was 2.79 times higher when both parietal and occipital injuries were involved compared with when they were not involved (95% CI, 1.25−6.23), and this finding was also statistically significant (P = .012) (Table 3).
Key features involved in thalamus L-sign injury compared with other thalamic injuries (nil and atypical)
Factors associated with the thalamic L-sign injury
The risk of experiencing a thalamus L-sign injury was 1.19 times higher when a brainstem injury was present compared with when it was not involved (95% CI, 1.09 −1.30), and this finding was statistically significant (P < .001). The risk of experiencing a thalamus L-sign injury was also higher when the frontal lobe, temporal lobe, and cerebellar injuries were all present compared with when they were not involved; however, this finding was not statistically significant at a 5% level of significance (Table 3).
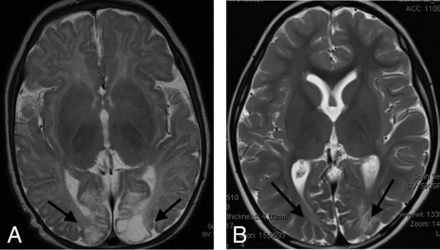
The second major group of patients studied included those with isolated hypoglycemic injury. This cohort comprised 13 term-gestation neonates with 9 males and 4 females. We noted that in all of these patients with confirmed pure HGI (without any documented hypoxic-ischemic injury), there was cerebral injury identified with gray and white matter involvement (as shown in Fig 3 and the Online Supplemental Data), which included collation of axial T2-weighted and FLAIR images (at the level of the thalamus) for 10 selected patients in this subgroup. In all these patients, we found cortical injuries, particularly involving the posterior watershed zones of the parietal and occipital lobes. The encephalomalacia seen in these brain areas on the chronic-phase imaging studies performed was largely indistinguishable from that seen in children who had HIBI. We did not identify anterior watershed cortical territory involvement in any of these 13 patients. Of critical importance, the posterior thalami were never injured in any of these 13 children with HGI. This is a key distinguishing feature from those children who had posterior watershed HIBI, in whom we consistently identified the thalamus L-sign.
Axial T2-weighted images in 2 children with proved neonatal hypoglycemia. There is bilateral occipital lobe encephalomalacia (arrows) related to hypoglycemic brain injury. Note the absence of any thalamic injury.
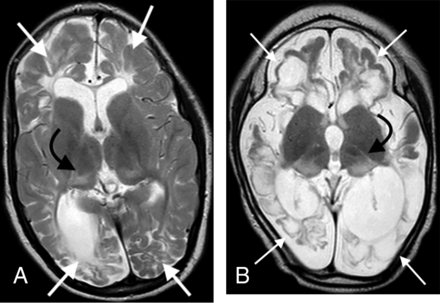
In the third group of 10 patients who sustained both HGI and HIBI, the thalamus L-sign was also observed in all patients on MR imaging studies acquired at the average age of 5 years. The axial T2-weighted images of these patients are compiled in the Online Supplemental Data. The blood glucose levels, Apgar scores at 1 and 5 minutes, as well as other clinical parameters of hypoxic-ischemic encephalopathy were documented in each child. When concomitant injury was incurred, there was an exaggerated final common pathway injury through the dorsal aspect of the thalami, which manifested as an L-shaped hyperintensity involving the pulvinar and lateral margin of the thalamus superimposed on features of watershed territory ischemic injury. This pattern of injury (common to all patients in this subgroup) is shown in 2 such examples in Fig 4. The degree of thalamic signal hyperintensity and volume loss was marked in all of these patients.
Combined hypoxic-ischemic and hypoglycemic brain injury in 2 children with documented neonatal encephalopathy. Note the exaggerated signal abnormality and thalamic volume loss (black arrows). There are multiple watershed areas (white arrows) demonstrating encephalomalacia change.
DISCUSSION
In partial, prolonged HIBI,6,8⇓-10 especially affecting the posterior and peri-Sylvian watershed zones, we have repeatedly shown involvement of the posterior and lateral aspects of the thalamus, which we believe to be a highly sensitive biomarker. This thalamus L-sign described here is consistently identified in children who have a perinatal, partial, prolonged pattern of HIBI. Additionally, we note that in our study, the thalamus L-sign was not observed in patients who had isolated, pure HGI without HIBI. We, therefore, propose the thalamus L-sign as a possible biomarker for HIBI of the partial, prolonged subtype, particularly when the posterior watershed territories have been involved. Furthermore, in patients who have endured combined hypoglycemia and hypoxia-ischemia, the phenomenon is exaggerated, likely due to the compounded lack of usable substrates for brain metabolism.12
The thalamus is a central hub serving to interconnect several brain structures to each other and to the cerebellum, spinal cord, and peripheral nervous system.13 Injuries to the thalamus provide an indirect indication of tract-based injuries to other parts of the brain, and by analyzing the involved substrates, we can infer a pattern of injury that can be assigned to a particular pathophysiologic process.14 Thalamic involvement in the HIBI of the central subtype injury pattern is well-documented 15,16 and typically demonstrates sparing of the pulvinar and lateral margin of the thalami.
The pulvinar or hockey stick sign has been linked to other disorders, including Fabry disease,17 a variant-type Creutzfeldt-Jakob disease,18 status epilepticus,19 and Wernicke encephalopathy.20 In contrast, in the cases described here, we found an inverted configuration to the hockey stick, with a characteristically repeated L-shape due to involvement of the posterior and lateral substrates of the thalamus rather than the paramedian nuclei. The thalamic nuclei involved, shown in Figs 2, 4, and 5, probably include the thalamic reticular nucleus (abutting the posterior limb of internal capsule), the pulvinar nucleus, and the lateral geniculate nucleus. Typically, these structures when contiguously involved lead to an L-shaped signal abnormality in the thalami. We postulated that these anatomic structures collaborate as a functional unit enabling the dorsal and ventral stream pathways that operate via multiple transthalamic connections.21 The dorsal stream pathway is responsible for recognition of objects in space and proposing or guiding subsequent actions.22 This stream begins with the visual (occipital) cortex identification of objects in the visual field, followed by spatial awareness of the object through parietal lobe connections. The ventral stream pathway also begins with visual cortex input, which passes through the lateral geniculate nuclei (especially the parvocellular layer) and from there onward to the temporal lobe (for limbic and memory connections) or to the parietal lobe via the dorsal stream (for accurate object location and motion initiation).23
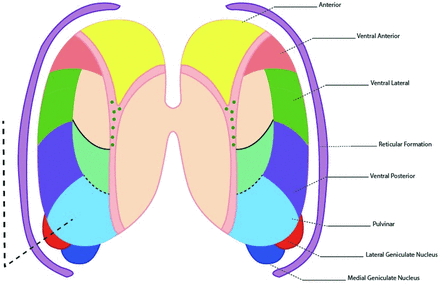
The key thalamic nuclei identified as components of the thalamus L-sign (dotted line) include the pulvinar, the lateral geniculate nucleus, and the reticular formation nuclei. Illustration by Neil Northey.
Jang et al24 performed a tract-based DTI analysis of the ascending reticular activating system in 14 children with HIBI. They demonstrated lower reticular activating system involvement in patients with impaired arousal. We believe that the posterolateral thalamic margin that we see in children with partial, prolonged HIBI includes, in part, involvement of the thalamic extension of the reticular activating system, which is located in this portion of the thalamus. Some of the hyperintensity may well be due to Wallerian degeneration change adjacent to the posterior limb of internal capsule secondary to the white matter involvement in the parietal, occipital, and peri-Sylvian regions, which pass through these corticospinal long tracts.9
We have found that all those with HIBI who demonstrated posterior watershed involvement either on its own or with peri-Sylvian watershed zone involvement also had a high correlation with a positive thalamus L-sign. In fact, all 59 patients (100%) who had partial, prolonged HIBI, all with watershed zone involvement, showed the thalamus L-sign.
In contrast, all those individuals with isolated anterior watershed involvement did not demonstrate the thalamus L-sign. When the anterior watershed was associated with other watershed territory involvement, we noted that the thalamus L-sign was often present, probably consequent to the more posterior cerebral injury. The posterior watershed territories supply axons that contribute to the projection fibers that traverse the centrum ovale, forceps major, and optic radiation. These white matter tracts and neural networks feed into the lateral geniculate nuclei and the pulvinar of the thalami. We found the risk of experiencing a thalamus L-sign injury to be 2.79 times higher when the parietal and occipital injuries were involved compared with when they were not involved (95% CI, 1.25−6.23), and this finding was statistically significant (P = .012) (Table 3). We speculate that the involvement of these thalamic nuclei may well reflect Wallerian degeneration along these tracts.
The patterns of brain injury seen in partial, prolonged HIBI have been found to be very similar to those of HGI, and there is considerable overlap in the injuries in these 2 entities.5,25 Most studies involve heterogeneous groups of children with concomitant or sequential hypoxic-ischemic and hypoglycemic brain injuries having been sustained. Wong et al5 demonstrated that specific imaging findings could be identified for both hypoglycemia and hypoxia-ischemia in term infants with neonatal encephalopathy. They showed an 82% positive predictive value for the radiologic diagnosis of hypoglycemic brain injury with selective posterior white matter and pulvinar edema the most predictive of clinical hypoglycemia. In contrast, none of the patients with hypoglycemia in our study demonstrated any signal abnormalities in the pulvinar or posterolateral thalami. We suspect that the changes described by Wong et al5 correspond to the third group in our study, in which a final common pathway injury was found. This suggestion is based on a listed limitation by the authors in that they were unable to separate HIBI from HGI in their cohort studied, and the described posterior thalamic injuries in that study were more likely representative of the mixed, final common pathway.
Tam et al25 showed an increased risk of injury to the corticospinal tracts in children who had perinatal hypoglycemia. Using multivariate logistic regression analysis to adjust for biomarkers of HIBI, the authors described an association between corticospinal tract injury and neonatal hypoglycemia. These patients, however, were not children with isolated hypoglycemia; many probably had HIBI, and the degree of corticospinal tract and other substrate injuries may largely be due to hypoxia-ischemia. An important conclusion raised by the same authors was the need for specific biomarkers to separate HIBI from HGI, something this study attempts to prove using the thalamus L-sign. Another limitation of that study25 was the variability in the timing of blood glucose measurement revealing hypoglycemia. This is a universal occurrence (which we also noted) with the timing of blood glucose monitoring and not a standardized practice. The variability in neonatal glucose levels warrants investigation, with a view to further establishing norms for the timing of the measurement thereof. In our study, we were fortunate in that all the patients that we identified with hypoglycemia had documented blood glucose levels either in the immediate neonatal period or in the first days of life.
This recommendation was echoed in the study by Basu et al,26 in which patients were referred to a central hospital from outlying hospitals and early glycemic measurements were not obtained in all patients. However, a strength noted in their study was the correlation between hypoglycemia and the predominant watershed pattern of brain injury, resonating the findings of previous studies.1 It has been shown that the combination of hypoglycemia and hypoxia-ischemia is associated with worse perinatal and long-term outcomes, including mortality.27,28 The upshot of anaerobic metabolism on a fetus that had hypoxia-ischemia is inefficient energy production and the glucose that is available is rapidly used, with attendant hypoglycemia and inadvertent lower energy output (1 glucose molecule yields 2 adenosine triphosphate molecules versus a 1:38 ratio in aerobic conditions).29 The difficulty has always been to separate the 2 entities.12 We have shown that by using this thalamus-L sign described here, we were able to possibly distinguish HIBI from pure HGI.
A limitation of our study was the retrospective evaluation of patients who were born some years earlier, with a lack of standardized recording of clinical information and timing of the MR imaging studies. Lack of complete medical records and blood test results (blood gas and glucose levels) was an exclusion criterion. All patients included here had available medical records. The possibility of having included other patients (if records were available) would have increased the subgroup numbers. It would possibly also improve the validity of the study with larger groups, and such prospective studies with timeous blood gas and glucose analyses would be encouraged.
CONCLUSIONS
The vicious interplay between hypoxia and hypoglycemia and their attendant secondary inflammatory cascades leads to a combined final common pathway injury, especially in patients whose mother had prolonged labor. The thalamus L-sign, we propose, is an indication of a partial, prolonged type of HIBI and occurs in patients who have endured additional HGI. In the patients presented here, who had documented, isolated, pure HGI without HIBI, the thalamus L-sign was not observed. We, therefore, introduce this sign as a possible biomarker for HIBI of the partial prolonged subtype, particularly when the posterior watershed territories have been involved. This phenomenon is exaggerated in patients with combined HGI and HIBI due to the compounded lack of usable substrates for brain metabolism.12 Future prospective studies, preferably with the MR imaging scans performed around the time of suspected perinatal injury, with clinical correlation of obstetric events (such as prolonged obstructive labor, uterine hyperstimulation, and other key factors) would serve to validate our study findings in the acute setting.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received December 24, 2021.
- Accepted after revision March 21, 2022.
- © 2022 by American Journal of Neuroradiology