Abstract
BACKGROUND AND PURPOSE: Neurologic manifestations in hereditary hemorrhagic telangiectasia include an increased incidence of brain abscesses and ischemic strokes due to paradoxic embolization in addition to a wide spectrum of symptoms and complications due to typical brain vascular malformations. Intracranial aneurysms are not part of this brain vascular malformation spectrum. The aim of this study was to determine their prevalence in patients with hereditary hemorrhagic telangiectasia.
MATERIALS AND METHODS: This was a single-center, retrospective study. Adult patients from the institutional Hereditary Hemorrhagic Telangiectasia registry with a definitive diagnosis of hereditary hemorrhagic telangiectasia and an available report or angiographic imaging study were included and reviewed to determine the intracranial aneurysm prevalence. In addition, the morphologic characteristics of intracranial aneurysms and possible associated risk factors were collected.
RESULTS: Two hundred twenty-eight patients were analyzed. Thirty-seven aneurysms in 33 patients (14.5%; 95% CI, 9.9%–19%) were found. The median diameter of intracranial aneurysms was 3.2 mm (interquartile range, 2.6–4.4 mm). No association between intracranial aneurysm and sex, age, or genetic background was noted. There were no subarachnoid hemorrhagic events due to intracranial aneurysm rupture.
CONCLUSIONS: Due to the high prevalence of intracranial aneurysms in adult patients with hereditary hemorrhagic telangiectasia, further studies regarding bleeding risks and monitoring should be addressed.
ABBREVIATIONS:
- ACVRL1
- activin receptor-like kinase 1 or ALK-1
- BVM
- brain vascular malformation
- ENG
- endoglin
- HHT
- hereditary hemorrhagic telangiectasia
- IA
- intracranial aneurysm
- IQR
- 25–75 interquartile range
- MADH4
- mothers against decapentaplegic drosophila homolog 4
- MMP
- matrix metalloproteinase
- TGFβ/BMP
- Transforming Growth Factorβ/bone morphogenetic protein
Hereditary hemorrhagic telangiectasia (HHT) is a multisystemic vascular disorder characterized by the presence of mucocutaneous telangiectasias and vascular malformations in organs including the central nervous system, liver, lungs, and the digestive tract.1 It is a rare autosomal dominant disease that affects 1 in 5000–8000 people worldwide.2,3 The diagnosis is based on the Curaçao criteria and/or genetic testing.3 The genes affected in HHT encode receptor proteins involved in the TGFβ/BMP signaling pathway, which plays an important role in angiogenesis and vascular remodeling.4 Mutations in the ENG or ACVRL1 genes are found in approximately 85% of individuals with HHT and up to 96% when the Curaçao criteria are strictly applied.5 Approximately 15% of patients diagnosed with HHT do not present with any of these gene mutations, suggesting that other genes might be involved. The MADH4 gene is mutated in patients with a combined syndrome of juvenile polyposis and HHT, representing 1%–3% of HHT cases.6
Neurologic manifestations of HHT include a higher incidence of brain abscesses and ischemic strokes due to paradoxic embolization through pulmonary arteriovenous fistulas, intracranial hemorrhages caused by BVMs, and mild or moderate psychomotor disturbances in some patients with manganese deposits in the basal ganglia.7⇓⇓-10 The spectrum of BVMs in HHT includes classic AVMs, capillary malformations, cavernous malformations, venous angiomas/developmental venous anomalies, vein of Galen malformations, telangiectasias, enlarged capillary-sized vessels, high-flow pial fistulas, and mixed malformations. Most classic presentations in HHT include multiple or single micro-AVMs or arteriovenous fistulas.7,10 The prevalence of BVMs is around 23%,11 with an estimated risk of hemorrhage that ranges from 0.27% to 0.46% annually.12 Screening with MR imaging is mandatory in children and adults, but a definitive diagnosis needs conventional angiography. Once a BVM is diagnosed, the management should be discussed with an expert vascular neurosurgery team.7,10,13
Intracranial aneurysms are acquired vascular lesions with an estimated prevalence ranging from 2% to 5% in the general population.14 The exact etiopathogenesis of intracranial aneurysms (IAs) still remains uncertain. It may result from congenital vascular wall defects, atheromatous disease, trauma or infectious emboli, or other systemic or local inflammatory processes.15 IAs are believed to be associated with the presence of AVMs,16 and IA development may be related to hemodynamic changes caused by the presence of shunting in AVMs.17
Although cases of IA have been reported in patients with HHT, the association between HHT and the existence of IA is still unclear.10 The main aim of this study was to describe the prevalence of IAs and their morphologic characteristics and explore possible risk factors associated with them.
MATERIALS AND METHODS
A descriptive/analytic cross-sectional study was designed and conducted at an HHT referral center in Buenos Aires, Argentina. Patients were eligible if they were older than 17 years of age, had definite HHT based on the presence of ≥3 Curaçao criteria (epistaxis: spontaneous and recurrent nasal bleeding; telangiectasias: multiple, at characteristic sites; visceral lesions: pulmonary, liver, gastrointestinal, cerebral, and spinal vascular malformations; family history: a first-degree relative with HHT according to these criteria) or a confirmed genetic test and had available angiographic studies (MRA, CTA, and/or cerebral angiography). The availability of these studies was defined as either images being uploaded to the hospital's medical record system or an available reliable imaging report with detailed data, in case the angiographic study was performed externally. The main outcome was to determine the prevalence of IAs within the study population. In addition, clinical and genetic factors would be analyzed to determine their possible association with an IA diagnosis. Data were obtained until December 2021 from the institutional HHT registry (ClinicalTrials.gov identifier: NCT01761981), which was started in 2012. This registry includes patients with confirmed HHT and/or who are in the process of being diagnosed. This study was approved by the local ethics review board, and each included patient consented to the use of data for research purposes. All the data analyzed in this study were properly anonymized.
Imaging Protocol and Analysis
CTA scans were obtained with a 64-detector-row CT system (Aquilion; Toshiba) or a 320-detector-row system (Aquilion One; Toshiba), including a nonenhanced phase and a contrast-enhanced phase, with 0.5-mm-thick sections every 0.3 mm, 0.641 pitch, 300 mAs, 120 kV, and 0.5-second scan time. For the contrast-enhanced phase, we used a 1-mL/kg dose of nonionic contrast medium (Iobitridol, Xenetix 350; Guerbet) delivered intravenously through an automatic injection pump at 4 mL/s. The DICOM data from the CT scanner were sent from a local computer to a Vitrea 2 workstation (Vital Images), where images are evaluated and 2D multiplanar reformations, 3D volume-rendering, maximum intensity projections, and minimum intensity projections were used. MRA images were obtained on 1.5T Achieva (Philips Healthcare), Magnetom Essenza (Siemens), or Magnetom Avanto (Siemens) magnets, or a 3T Ingenia (Philips Healthcare) system. The protocol included T2, FLAIR, gradient recalled-echo, DWI, and T1-weighted images in an axial plane, axial TOF volume, and contrast-enhanced T1-weighted spoiled gradient echo sagittal volume. Cerebral DSAs were performed using Integris V5000 (Phillips Healthcare), BV 300 (Phillips Healthcare), or Artis Zeego (Siemens) systems. The angiographic studies that were part of the initial clinical assessment of the included patients were retrospectively reviewed by 2 senior neuroradiologists. In case of disagreement, consensus was reached by a third neuroradiologist. The presence and number of aneurysms per patient and their imaging characteristics including geometric measurements (width, height, and neck diameter) and anatomic location were registered.
Other Registered Variables
We registered key clinical variables: age, sex, Curaçao criteria, the presence of other vascular malformations in each enrolled patient, and the results of mutations on target genes (ENG, ACVRL1, and MADH4) when available. The presence of other risk factors associated with IAs in the general population such as hypertension and tobacco use were also taken into account.
Statistical Analysis
Continuous variables are described as the mean and SD or median and interquartile range (IQR) according to distribution. Categoric variables are described as absolute frequency and percentage. The IA prevalence was determined as the proportion of patients having at least 1 IA within the total population of patients with confirmed HHT who had at least 1 suitable angiographic study available for revision. For analyzing the association between baseline clinical and genetic characteristics with the outcome, categoric data were compared using the Fisher exact test, and continuous data were compared using the Mann-Whitney test. All statistical analyses were performed using STATA, Version 14 (StataCorp). One- and 2-sided P values < .05 were considered statistically significant.
RESULTS
Of the 707 patients currently enrolled in the registry, 566 had confirmed HHT. Of these, 228 (40.3%) subjects had an angiographic study and were finally included in the study. Reliable imaging reports were the only source of data in 38 (16.7%) of the included patients. Female patients represented 61.8% of the included population (Online Supplemental Data). The median age was 49.0 years (IQR, 38.4–64.1 years). Demographic characteristics are detailed in Table 1.
Population characteristics
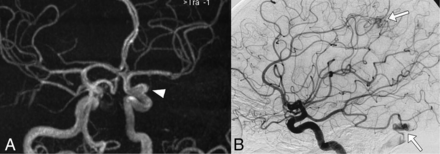
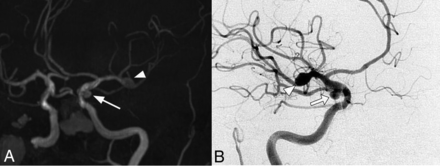
Thirty-seven aneurysms were identified in 33 patients (14.5%; 95% CI, 9.9%–19%), with 2 patients having 2 and another single patient having 3 IAs. The most frequent locations of IAs were the following: 9 in the middle cerebral artery, 9 in the carotid intracavernous segment, and 8 in the ophthalmic segment of the ICA. The median diameter of IAs was 3.2 mm (IQR, 2.6–4.4 mm). Further IA characteristics are mentioned in Table 2. Angiographic images of IAs from 2 different patients are shown in Figs 1 and 2.
Cerebral MRA (A) and DSA (B) images of a 39-year-old female patient that meet 4 of 4 Curaçao criteria show an ophthalmic segment of a left carotid artery aneurysm (arrowhead) and AVMs in the parietal and occipital contralateral lobes (arrows).
Cerebral MRA (A) and DSA (B) images of a 78-year-old female patient that meet 4 of 4 Curaçao criteria show a right MCA bifurcation aneurysm (arrowhead) and another one located in the right posterior communicating artery (arrow).
Intracranial aneurysm characteristics
AVMs were found in 39 patients; within this subgroup, 7 patients also had IAs, with no statistical association between both vascular malformations. Only 2 IAs were located in the same vascular territory as the AVM. Furthermore, among those patients who had available images for studying aneurysms outside the CNS, no association between IAs and aneurysms (mostly splanchnic) in other body segments was found. There were no differences in either age, sex, Curaçao criteria, hypertension, or tobacco use between patients with and without IAs. Genetic results were available in 81 patients (35.5%), with 39 patients having ENG mutations, 25 having ACVRL1 mutations, and 10 with MADH4 mutations. In 7 patients, genetic tests were noninformative. No statistically significant association was found between patients with and without IAs regarding the distribution of genetic results (Table 3).
Bivariate analysis between baseline variables and intracranial aneurysms
DISCUSSION
A high prevalence of IAs among patients with HHT was found in this study. The IA prevalence was also increased in patients with a family history of IA18 and in certain inherited diseases, including autosomal dominant polycystic kidney disease and some connective tissue disorders.19 A retrospective study by Kim et al20 showed that the prevalence of IAs was as follows: 14% among patients with Marfan syndrome, 12% among patients with Ehlers-Danlos syndrome, 11% among patients with neurofibromatosis type 1, and 28% among patients with Loeys-Dietz syndrome. The Loeys-Dietz syndrome is associated with genetic mutations, also involving the TGF-β/BMP pathway, especially in the SMAD2/3 proteins, a pathway that is also altered in HHT.20⇓-22 Moreover, the combined syndrome of juvenile polyposis and HHT syndrome, caused by MADH4 mutations, is also associated with aortic root aneurysms and joint hypermobility.23,24
Numerous reports describe the presence of IAs in patients with HHT,25⇓⇓⇓⇓⇓⇓⇓-33 and many authors include HHT in a group of hereditary diseases that present with a higher prevalence of IAs, such as Marfan, Ehlers-Danlos, and polycystic kidney syndromes and neurofibromatosis,19,34,35 but these studies do not specifically analyze the prevalence of IAs in patients with HHT in a quantitative manner. Several authors have studied the neurologic manifestations of HHT.11,36⇓-38 These researchers describe multiple kinds of BVMs and, second, mention the frequency of IAs, such as Brinjikji et al,38,39 whose findings indicated that the prevalence of IAs is around 2%, similar to that of the general population. There is a specific study that looked for the prevalence of aneurysms in all locations in HHT, which reported a higher prevalence of IAs of around 4%.40 The number of aneurysms in these populations might have been underestimated due to the lack of angiographic studies to evaluate many of these patients.
The prevalence of IAs in the present HHT cohort was 14.5%, whereas the reported prevalence in the general population is close to 3%–5%.18 Most of the aneurysms were small, with a predilection for the anterior circulation, similar to those observed in the general population.15 These findings could correlate with the vascular malformations, which these patients have in multiple organs, even aneurysms in the splanchnic circulation. Considering that BVMs are more frequent in patients with HHT having the ENG mutation,41 a possible link between IAs and genotypes arises, but no statistically significant association was found.
The coexistence of IA and brain AVMs in patients without HHT is estimated to be between 5% and 20%.42 Although the pathogenesis of IAs in these patients is not fully understood, it is believed that it may be related to hemodynamic factors triggered by the presence of a shunt in the AVM.17 AVMs and IAs were both found in 7 patients, but only 2 were in the same vascular territory as the AVM, which may be interpreted as flow-related. Even subtracting these 2 patients, the prevalence would still be high, so the increased prevalence of IAs in HHT could be explained by vascular alterations caused by mutations in genes of the TGFβ/BMP pathway mentioned above. There is evidence pointing to inflammation as a mechanism of aneurysm formation in patients without HHT. The process begins with a hemodynamic or inflammatory insult, with a local increase in matrix metalloproteinases (MMPs) released by macrophages and mast cells, with intimal migration and shape changes of the smooth-muscle cells.43 In HHT, a state of chronic inflammation of the vascular wall, induced by an imbalance between pro- and anti-inflammatory factors of mononuclear cells, has been widely reported. This would explain one of the possible biologic bases to suspect a higher incidence of IAs in HHT.44 In addition, endoglin is involved in cell migration, proliferation, and extracellular matrix remodeling, mechanisms that involve MMPs and macrophages.44,45 Furthermore, endoglin and TGFβ/BMP are involved in inflammation and the tissue-repair processes as well as in angiogenesis. The vascular wall of AVMs in HHT is abnormal and lacks organized mural cell layers such as pericytes and smooth-muscle cells.46 Finally, drugs that inhibit MMPs such as doxycycline are proposed for the treatment of epistaxis in HHT and unruptured IAs.43,47
The present study has research limitations to be acknowledged. First, it is a retrospective study and, thus, though only patients with angiographic studies were included, not all of them were evaluated with the same angiographic imaging technique, and alternative modalities have different sensitivities and specificities to detect IAs. In addition, a small proportion of these patients had a reliable imaging report but no available image for us to re-evaluate. Along the same lines, there were missing data regarding other risk factors for aneurysm development in some patients. Patient genotypes were not available for the entire cohort, and the relationship between IAs and mutations could not be fully studied; therefore, the lack of an association found should be viewed with caution until further studies are developed. Only adult patients were enrolled, as for other BVMs, the prevalence could be similar in the pediatric population, but this issue should be addressed in future studies. Finally, because this study does not have a control group and there is a lack of regional data on IA prevalence, these results have been compared with those reported in other populations.
Patients with HHT are usually evaluated for BVMs with contrast-enhanced MR imaging; however, the results found in this study may suggest adding an angiography sequence to the current screening methodology in search of IAs. The largest meta-analysis on IA rupture risk included data from 8382 patients. This study identified 6 independent risk factors for aneurysm rupture: older than 70 years of age, hypertension, previous SAH from another aneurysm, the size and site of the aneurysm, and the patient's geographic region. The subsequently developed score for prediction of aneurysm rupture risk is based on these 6 key risk factors and provides absolute estimates for a 5-year rupture risk, ranging from 0.3% to ≥15%.48 There were no SAH events in this cohort due to IA rupture, and all patients are under close follow-up and/or have already been treated according to the usual standards of care. Although there is no prospective information about the rate of rupture of IA in patients with HHT, medical follow-up on these patients should be considered to prevent SAH with its high morbimortality rate.
CONCLUSIONS
The present study suggests an increased prevalence of IA in HHT compared with the general population. Considering the morbimortality associated with aneurysm rupture, these findings highlight the importance of conducting larger studies in the future; including pediatric population, evaluating the etiopathogenesis of IAs associated with HHT and discussing the clinical relevance and usefulness of different detection methods, follow-up and currently available treatments.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 30, 2022.
- Accepted after revision March 17, 2022.
- © 2022 by American Journal of Neuroradiology