Abstract
BACKGROUND AND PURPOSE: Functional MR imaging is widely used for preoperative language assessment in candidates for resective neurosurgery. Language mapping paradigms that are adaptive to participant performance have the potential to engage the language network more robustly and consistently, resulting in more accurate functional maps. The aim of the current study was to compare two adaptive paradigms with the recommended language mapping paradigms that constitute the current standard of care.
MATERIALS AND METHODS: Seventy-three patients undergoing fMRI for language lateralization and/or localization completed an adaptive semantic matching paradigm, an adaptive phonological judgment paradigm, and two standard paradigms: sentence completion and word generation. The paradigms were compared in terms of the degree to which they yielded lateralized language maps and the extent of activation in frontal, temporal, and parietal language regions.
RESULTS: The adaptive semantic paradigm resulted in the most strongly lateralized activation maps, the greatest extent of frontal and temporal activations, and the greatest proportion of overall satisfactory language maps. The adaptive phonological paradigm identified anterior inferior parietal phonological encoding regions in most patients, unlike any of the other paradigms.
CONCLUSIONS: The adaptive language mapping paradigms investigated have several psychometric advantages compared with currently recommended paradigms. Adoption of these paradigms could increase the likelihood of obtaining satisfactory language maps in each individual patient.
ABBREVIATION:
- LI
- lateralization index
fMRI is widely used for presurgical language mapping in patients who are candidates for resective surgery for epilepsy, brain tumors, and vascular malformations.1-3 One goal of presurgical language mapping is to determine language lateralization, and fMRI compares favorably with the invasive Wada test for this purpose.4-7 A second goal is to identify indispensable language regions to aid in tailoring surgical margins; fMRI is also widely used for this purpose,3 though its validity has not been established.8 Many different paradigms are used for language mapping (eg, sentence completion, word generation, and so forth), and numerous studies have compared the validity and reliability of various sets of paradigms.1,9-13 In 2017, a task force of American Society of Functional Neuroradiology members reviewed this literature and recommended sentence completion and word generation as the first two tasks that should be performed in adult patients.2 However, this recommendation was based primarily on practical considerations, including widespread existing use, rather than a detailed assessment of the psychometric properties of different paradigms that have been proposed.
Recently, we have described a pair of semantic and phonological language mapping paradigms that are adaptive to patient performance; that is, the difficulty of the tasks is dynamically modulated on the basis of the patient’s responses.13-15 The motivation for developing these adaptive paradigms was to perform language mapping in individuals with aphasia, whose language deficits may preclude performance of many tasks used in clinical practice. We found that the adaptive tasks could be performed successfully by most individuals with aphasia and had superior psychometric properties compared with several other tasks in individuals with aphasia and neurologically healthy controls.13-15 The adaptive nature of the tasks entails that they remain challenging yet feasible at all times, thus tightly constraining participants’ cognitive states, resulting in robust recruitment of the language network and good test-retest reproducibility.
The aim of the present study was to investigate the utility of these adaptive semantic and phonological language mapping paradigms for presurgical language mapping. Most candidates for resective surgery have no language deficits or mild language deficits, but occasionally, patients present with moderate or even severe aphasia. The ability of the adaptive paradigms to reliably identify language areas in patients with and without language deficits suggests that they have strong potential to be appropriate for this population. We administered both paradigms, along with the currently recommended sentence completion and word generation paradigms, to map language regions in patients with epilepsy, brain tumors, or vascular malformations who were referred for presurgical language mapping. We operationalized success as the ability of each paradigm to yield lateralized language maps that included activation of known frontal, temporal, and anterior parietal language regions.
MATERIALS AND METHODS
Patients
All patients referred for presurgical language mapping at Vanderbilt University Medical Center between September 2019 and December 2021 were considered for inclusion. During this time period (and continuing), it was our practice to perform all 4 language mapping paradigms whenever possible for clinical purposes.
A total of 73 patients provided written informed consent to participate in the study. Demographic information is provided in Table 1. Patients were diagnosed with epilepsy (n = 55), tumor (n = 16), AVM (n = 1), or a cavernous malformation (n = 1). Forty-two of 73 patients (58%) reported some degree of language impairment. Language deficits were mild in most cases. The sample was consecutive for the first half of the study period and constituted approximately every second patient during the second half of the study period, with inclusion determined by scheduling and not by any patient factor. No patients declined consent. A minority of patients were not asked to consent because language mapping was expected to be based on <4 paradigms due to time constraints; this was most often the case when extensive motor mapping was required as well.
Characteristics of the 73 participants
The study was approved by the institutional review board at Vanderbilt University Medical Center.
Language Mapping Paradigms
Each of the 4 paradigms involved a simple 2-condition block design with 6 blocks per condition and 20 seconds per block, for a total scan time of exactly 4 minutes.
The adaptive semantic paradigm has been described in detail previously,13 but in brief, there were 2 conditions: a semantic matching task and a perceptual matching task. In the semantic matching condition, participants saw 2 words in the middle of the screen, one above the other, and were instructed to press a button if the words were semantically related (eg, boy-girl) and to do nothing if the words were unrelated (eg, walnut-bicycle). In the perceptual matching condition, participants saw 2 strings of symbols in the middle of the screen, one above the other, and were instructed to press a button if the 2 strings were identical (eg, [ΔΘδЂϞ-ΔΘδЂϞ]) and to do nothing if they were different (eg, [ΔΘδЂϞ-ϞΔƕƘΔ]). Each condition had 7 levels of difficulty, and participants progressed to the next level every time they made 2 consecutive correct responses and stepped back 2 levels every time they made an error. Difficulty was modulated in the semantic condition by manipulating word frequency, concreteness, length, age of acquisition, degree of semantic relatedness, and presentation rate and, in the perceptual condition, by manipulating the degree of similarity of mismatching items and presentation rate.
The adaptive phonological paradigm has also been described previously.15 The language condition was a rhyme judgment task in which participants saw 2 pseudowords in the middle of the screen, one above the other, and were instructed to press a button if the pseudowords rhymed (eg, mulky-tulkie) and to do nothing if they did not (eg, shofy-sheffy). Difficulty was modulated by manipulating pseudoword length, orthographic transparency, stress pattern, and presentation rate. The perceptual control condition was the same as for the semantic paradigm.
Both adaptive paradigms were implemented in Matlab (MathWorks) using Psychtoolbox16,17 and are freely available online at https://langneurosci.org/alm.
The sentence completion paradigm was implemented as recommended by the American Society of Functional Neuroradiology,2 and the stimuli were downloaded from their Web site (https://www.asfnr.org/paradigms). In the language condition, participants saw 4 sentences per block (5 seconds each) with a blank space instead of the final word and were instructed to silently think of one or more words that could fit into the blank. In the control condition, participants saw scrambled letters matching the sentences in the number of characters and placement of spaces and were instructed to do nothing.
The word generation paradigm was also presented as recommended,2 with the stimuli provided. In the language condition, participants saw a letter in the middle of the screen (2 per block, 10 seconds each) and were instructed to think of as many words as possible that start with that letter. In the control condition, participants saw symbols in the middle of the screen and were instructed to do nothing.
Before scanning, patients were trained on all 4 paradigms by 1 of the 3 authors. Training items were not repeated in the scanning session. For the adaptive paradigms, each trial type was demonstrated and discussed; then patients practiced a few blocks with actual task timing and adaptive features.13,15 The sentence completion and word generation paradigms were practiced out loud to ensure task compliance; then the patient was instructed to perform the tasks silently in the scanner. In the scanner, the order of the 4 paradigms was counterbalanced across participants, to ensure that results were not confounded by presentation order. We cycled through 4 presentation orders so that each of the 4 paradigms was equally likely to be performed first, second, third, or fourth.
Neuroimaging
MR imaging data were acquired on a Philips Achieva 3T scanner (n = 57) or a Philips Ingenia Elition 3T scanner (n = 16) (Philips Healthcare) with 32-channel head coils at Vanderbilt University Medical Center. All paradigms were controlled by a laptop computer (Thinkpad T490s; Lenovo) running Matlab (Mathworks) Version R2019a and Psychtoolbox Version 3.0.16.16,17 Visual stimuli were presented using a projector and a screen in front of the bore (n = 57) or on a monitor positioned behind the bore (n = 16), either of which patients viewed through a mirror mounted on the head coil. In the adaptive paradigms, patients responded on an MR imaging–compatible button box connected to the laptop.
For each of the 4 language mapping paradigms, sequences of T2*-weighted blood oxygen level–dependent echo-planar images were collected with the following parameters: 120 volumes + 5 initial volumes discarded; 35 or 36 axial slices in interleaved order; slice thickness = 3.5 mm with a 0.5-mm gap; FOV = 240 × 240 mm; matrix = 64 × 64; TR = 2000 ms; TE = 35 ms; flip angle = 78°; sensitivity-encoding factor = 2; voxel size = 3.75 × 3.75 × 4 mm.
For anatomic reference, T1-weighted (voxel size = 1 × 1 × 1 mm) and FLAIR images that were coplanar with the functional images (voxel size = 0.5 × 0.5 × 4 mm) were also acquired.
The imaging data were processed using standard methods as described previously.13,15 The functional data were first preprocessed with AFNI: Slice timing and head motion were corrected, then the data were detrended and smoothed with a 6-mm full width at half maximum Gaussian kernel. Next, independent-component analysis was performed using the FSL tool MELODIC. Noise components were manually identified and removed using fsl_regfilt. All paradigms were modeled with boxcar functions convolved with a hemodynamic response function and fit to the data with the FMRISTAT program fmrilm. The 6 head-motion parameters were included as covariates, as were time-series from white matter and CSF regions to account for nonspecific global fluctuations and 3 cubic spline temporal trends. The T1-weighted anatomic images were warped to Montreal Neurological Institute space using unified segmentation in SPM12. Functional images were coregistered with structural images via coplanar FLAIR images using SPM and warped to Montreal Neurological Institute space. Individual activation maps were thresholded with a 5% relative threshold18 and a minimum cluster extent of 2 cm3, as described previously.13,15
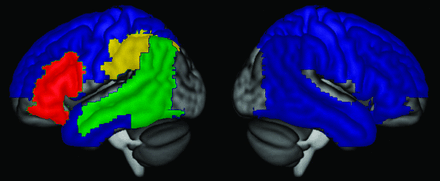
To compare the paradigms in terms of their ability to reveal hemispheric dominance, we calculated lateralization indices (LIs) in an extensive bilateral ROI (Fig 1) comprising the inferior, middle, and superior frontal gyri, supplementary motor area, precentral and postcentral gyri, all of the lateral parietal lobe, and all of the temporal lobe (lateral and medial) except for the dorsal (auditory) part of the superior temporal gyrus. This same a priori ROI has been used previously.13
ROIs. LIs were calculated on the basis of activation throughout the wide region shown in blue (or any of the other colors), while sensitivity was determined on the basis of activations in frontal (red), temporal (green), and anterior parietal (yellow) language regions.
Frontal, temporal, and anterior parietal language ROIs were defined to assess the sensitivity for detection of activation in these 3 language regions (Fig 1). These ROIs were defined in the dominant hemisphere, according to our clinical judgment of language lateralization based on all 4 paradigms, or in the left hemisphere in patients with bilateral language. Regions were deemed activated when there was ≥4 cm3 activation within the region. The frontal ROI was defined as the inferior frontal gyrus, as defined previously13,15 on the basis of the Automated Anatomical Labelling atlas.19 The temporal ROI was defined previously13,15 as the ventral part of the superior temporal gyrus, the middle temporal gyrus, and the angular gyrus; in this study, this ROI was expanded to also include the lateral and posterior parts of the inferior temporal and fusiform gyri (|x| ≥ 38, y ≤ −38). The anterior parietal ROI was defined as the supramarginal gyrus and the inferior parietal lobule, as previously defined;15 a language region within this territory has been shown to be critical for phonological encoding.15,20-23
Language maps were defined as satisfactory when they met 3 conditions: 1) |LI| ≥ 0.25, in the correct direction or for patients with bilateral language,−0.25 < LI <0.25; these cutoffs are based on those proposed by Janecek et al;5 2) frontal activation of ≥4 cm3 in the dominant hemisphere (left hemisphere for patients with bilateral language); and 3) temporal activation of ≥4 cm3 in the dominant hemisphere (left hemisphere for patients with bilateral language).
Test-retest reproducibility was assessed in a preliminary manner by splitting each run in half, analyzing the 2 halves separately, and calculating the Dice coefficient of similarity24 between the 2 resultant activation maps. Head motion was compared between paradigms by calculating the mean framewise displacement for each run.
LIs, activation extents, Dice coefficients, and head-motion measures were compared among paradigms using repeated measures ANOVAs and post hoc paired t tests. The proportions of satisfactory language maps were compared with a χ2 test, followed by post hoc Fisher exact tests.
RESULTS
The mean accuracy on the adaptive semantic task was 84.1% (SD, 5.0%) (range, 58.3%–95.0%), indicating that all patients performed the task above chance. Accuracy on the adaptive phonological task was 77.8% (SD, 9.5%) (range, 33.3%–91.1%), indicating that most but not all patients performed the task above chance. The mean difficulty level of items presented was 4.1 (SD, 2.8) (on a 7-point scale) (range, 1.7–6.1) for the semantic task and 2.8 (SD, 1.1) (range, 1.2–6.2) for the phonological task; these means are about 1 point lower than previously observed in neurologically healthy individuals.15 Because the sentence completion and word generation paradigms were performed covertly, it was not possible to evaluate performance, but all patients were able to perform both paradigms during prescan training.
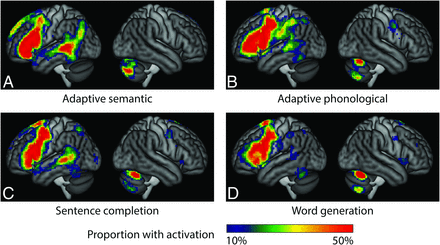
On the basis of our clinical judgments, taking into account all 4 paradigms, language was localized to the left hemisphere in 67 patients, localized to the right hemisphere in 4 patients, and bilaterally distributed in 2 patients. The brain regions activated by each of the 4 paradigms are shown in Fig 2, in which activations for the 4 patients with right-lateralized language have been mirror-reversed around the midline. All 4 paradigms yielded extensive activation in the inferior frontal lobe. In the posterior temporal lobe, the most robust language activation was observed for the adaptive semantic paradigm, followed by the sentence completion paradigm. In contrast, the adaptive phonological paradigm activated temporal language areas in many but not all patients, while the word generation paradigm yielded temporal activation in even fewer patients. The anterior parietal region was activated by the adaptive phonological paradigm in most patients, but rarely by the other 3 paradigms. Finally, all 4 paradigms activated the contralateral cerebellum.
Activation maps for each paradigm. The color map indicates the number of individual patients with activation, with a whole-brain ROI, relative threshold of 5%, and minimum cluster extent of 2 cm3. Activation maps for patients with right-hemisphere dominance were flipped for these maps. A, Adaptive semantic. B, Adaptive phonological. C, Sentence completion. D, Word generation.
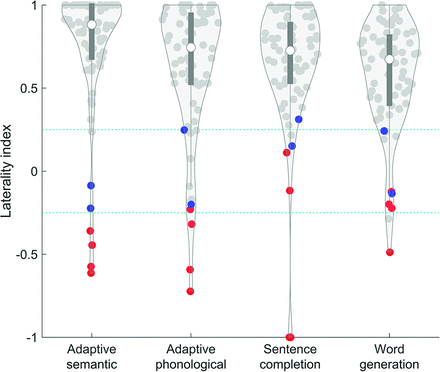
All 4 paradigms revealed satisfactory lateralization determinations (ie, |LI| ≥ 0.25, in the correct direction) in most patients (adaptive semantic: 72 of 73; adaptive phonological: 67 of 73; sentence completion: 71 of 73; word generation: 69 of 73) (Fig 3). However, the 4 paradigms did differ in the degree to which language activations were lateralized (F(3,216) = 12.029, P < .001). Note that for the 4 patients with right-lateralized language, LIs were negated in the statistical analysis (but not in the figure). Post hoc tests indicated that the adaptive semantic paradigm (mean |LI| = 0.80 ± 0.25) yielded higher LIs than the other 3 paradigms (all, t(72) ≥ 3.05; all P ≤ .003). The adaptive phonological paradigm (mean |LI| = 0.68 [SD, 0.31]) did not differ from the 2 standard paradigms (all P ≥ .060), while the sentence completion paradigm (mean |LI| = 0.71 [SD, 0.25]) yielded higher absolute LIs than the word generation paradigm (mean |LI| = 0.60 [SD, 0.27], P = .003).
Lateralization indices by paradigm. Violin plots show the distribution of patients. Red dots indicate patients with right-hemisphere language, and blue dots indicate patients with bilateral language lateralization. Teal dotted lines show cutoffs for lateralization categories.
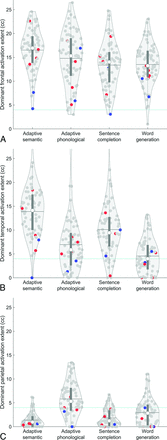
All 4 paradigms activated the frontal language region of the dominant hemisphere in most patients (adaptive semantic: 73 of 73; adaptive phonological: 70 of 73; sentence completion: 72 of 73; word generation: 72 of 73) (Fig 4A). However, the 4 paradigms differed in the extent of dominant-hemisphere frontal activation (F(3,216) = 12.43, P < .001). Post hoc tests indicated that the adaptive semantic paradigm produced the greatest extent of activation (all P ≤ .005), followed by the adaptive phonological paradigm (all P ≤ .031), then the 2 standard paradigms, which did not differ from each other (P = .83).
Sensitivity for identifying language regions in the dominant hemisphere. Violin plots show the extent of activation in each region for each paradigm. Red dots indicate patients with right-hemisphere language, and blue dots indicate patients with bilateral language lateralization. Horizontal lines show means. Teal dotted lines show cutoffs for assessment of sensitivity. A, Inferior frontal language region. B, Posterior temporal language region. C, Anterior parietal language region.
The 4 paradigms differed markedly in their capacity to activate the dominant-hemisphere temporal language region (adaptive semantic: 72 of 73; adaptive phonological: 52 of 73; sentence completion: 64 of 73; word generation: 37 of 73) (Fig 4B). The differences in the extent of activation in this region were statistically significant (F(3,216) = 90.06, P < .001). Post hoc tests indicated that the adaptive semantic paradigm produced the greatest extent of activation (all P < .001), followed by the sentence completion paradigm (all P < .001), then the adaptive phonological paradigm (P < .001), and finally, the word generation paradigm.
The 4 paradigms also differed markedly in their capacity to activate the dominant-hemisphere anterior parietal language region involved in phonological encoding (adaptive semantic: 7 of 73; adaptive phonological: 48 of 73; sentence completion: 9 of 73; word generation: 22 of 73) (Fig 4C). The differences in the extent of activation in this region were statistically significant (F(3,216) = 70.12, P < .001). Post hoc tests indicated that the adaptive phonological paradigm produced the greatest extent of activation (all P < .001), followed by the word generation paradigm (all P < .001), then the sentence completion paradigm (P = .031), and last, the adaptive semantic paradigm.
Finally, the paradigms were compared in terms of the number of patients for whom overall satisfactory language maps were obtained, ie, correctly lateralized with dominant-hemisphere frontal and temporal activations each exceeding 4 cm3. These proportions differed significantly across the 4 paradigms (χ2[3] = 50.14, P < .001) (Table 2). The adaptive semantic paradigm met these 3 criteria in the most patients (71 of 73, 97%), followed by the sentence completion paradigm (63 of 73, 86%), the adaptive phonological paradigm (49 of 73, 67%), and the word generation paradigm (37 of 73, 51%). All pair-wise differences were significant (Fisher exact test, all P ≤ .031), except for the difference between the phonological and word generation paradigms (P = .064).
Proportions of patients with satisfactory language maps
Neither test-retest reliability (F(3,216) = 1.99, P = .12) nor head motion (F(3, 216) = 0.97, P = .41) differed across the 4 paradigms. The relative performance of the 4 paradigms was maintained across different choices of analysis parameters, including ROIs, absolute or relative voxelwise thresholds, and cluster-extent thresholds (Online Supplemental Data).
DISCUSSION
Our data indicate that the adaptive semantic paradigm has the strongest psychometric properties of the 4 paradigms investigated, and for most purposes, it is most likely to result in satisfactory maps of individual patient language networks. For determination of language lateralization and for identification of the frontal language region of the dominant hemisphere, all 4 paradigms performed well in most patients, and the advantages of the adaptive semantic paradigm, though statistically significant, were modest. However, for identification of the temporal language region of the dominant hemisphere, the adaptive semantic paradigm performed markedly better than the other 3 paradigms, and consequently, it was the paradigm most likely to produce language maps that were satisfactory overall.
In our clinical practice, we continue to use all 4 paradigms whenever possible. A panel of tasks provides multiple fallback possibilities, and several studies have demonstrated the advantages of panels of tasks relative to single tasks.11,25,26 However, there are often situations in which time is limited and paradigms must be prioritized over one another. For example, patients who are at risk of experiencing a seizure in the scanner or patients who are claustrophobic may not be able to complete a full panel of tasks. On the basis of our data, we recommend that the adaptive semantic paradigm be administered first, so if a patient cannot complete a full panel of tasks for any reason, the likelihood of obtaining a satisfactory language map is maximized.
The only exception is in patients with tumors or epileptogenic foci in the parietal lobe, for whom there is an interest in localizing language regions with respect to the intended resection site. The adaptive phonological paradigm excelled at activating the anterior parietal language region, which is involved in phonological encoding.20-23 This region was activated by this paradigm in about two-thirds of the patients, greatly exceeding the other 3 paradigms, which usually do not reveal this language region. Parietal resections are relatively uncommon relative to temporal and frontal resections, but they are certainly sometimes indicated. In these patients, the adaptive phonological paradigm should be prioritized. Because sensitivity for this region was only 66%, we recommend that the paradigm be repeated more than once because it is likely that additional data would increase sensitivity.15 Other paradigms such as syllable counting, which also activates this region, could also be considered.15,20
There are several design factors that may account for the good performance of the adaptive paradigms. First, the adaptive nature of the tasks ensures that participants are always performing tasks that are challenging, yet within their abilities. This feature means that the language network is strongly driven during the language condition, while other brain regions are robustly recruited during the control condition, thus maximizing differences between the conditions. Second, the control conditions are tightly matched to the language conditions for task demands, thus avoiding spurious activations due to visual processing, decision-making, and so forth. Third, the combination of active decision-making and comprehension is well-suited to activating both frontal and temporal language areas in the case of the semantic task,27 while reading and phonological encoding of pseudowords place a heavy load on the phonological system in the case of the phonological task.15 Another advantage of the adaptive tasks is that they allow observation of responses and assessment of accuracy, which can be helpful in the interpretation of atypical activation maps.
There are two practical considerations in using the adaptive paradigms. First, an MR imaging–compatible button box is required to collect the responses that are used to select subsequent stimuli. Second, the adaptive language mapping software depends on Matlab and Psychtoolbox, the former being expensive and the latter requiring modest technical expertise to install correctly. A stand-alone version of the adaptive language mapping software is a priority for future work.
The present study has one noteworthy limitation, which is that successful language mapping was defined relative to expected patterns (lateralization and identification of known language regions), rather than with reference to clinical outcomes after surgery.28 Studies of outcomes in relation to alternative methods of localizing eloquent regions are generally difficult to perform because it would not be ethical to resect brain regions that one method (but perhaps not another) indicated were critical for language function. Therefore, the present evidence may be the strongest that is feasible to obtain in practice.
CONCLUSIONS
We found that the adaptive semantic paradigm is most likely to yield satisfactory language maps compared with the other paradigms investigated, and we, therefore, recommend this paradigm in most circumstances. When parietal localization is of particular concern, we recommend the adaptive phonological paradigm. If there is sufficient time, then we recommend that a panel of all 4 paradigms be used.
Acknowledgments
We thank all patients who took part in this research, and 2 anonymous reviewers for their constructive suggestions.
Footnotes
This work was supported by the National Institute on Deafness and Other Communication Disorders, R01 DC013270; and the National Institute of Neurological Disorders and Stroke, R01 NS110130, R01 NS108445.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 1, 2022.
- Accepted after revision July 12, 2022.
- © 2022 by American Journal of Neuroradiology