Abstract
BACKGROUND AND PURPOSE: Acute leptomeningeal collateral flow is vital for maintaining perfusion to penumbral tissue in acute ischemic stroke caused by large-vessel occlusion. In this study, we aimed to investigate the clinically available indicators of leptomeningeal collateral variability in embolic large-vessel occlusion.
MATERIALS AND METHODS: Among prospectively registered consecutive patients with acute embolic anterior circulation large-vessel occlusion treated with thrombectomy, we analyzed 108 patients admitted from January 2015 to December 2019 who underwent evaluation of leptomeningeal collateral status on pretreatment CTA. Clinical characteristics, extent of leukoaraiosis on MR imaging, embolic stroke subtype, time of imaging, occlusive thrombus characteristics, presenting stroke severity, and clinical outcome were collected. The clinical indicators of good collateral status (>50% collateral filling of the occluded territory) were analyzed using multivariate logistic regression analysis.
RESULTS: Good collateral status was present in 67 patients (62%) and associated with independent functional outcomes at 3 months. Reduced leukoaraiosis (total Fazekas score, 0–2) was positively related to good collateral status (OR, 9.57; 95% CI, 2.49–47.75), while the cardioembolic stroke mechanism was inversely related to good collateral status (OR, 0.17; 95% CI, 0.02–0.87). In 82 patients with cardioembolic stroke, shorter thrombus length (OR, 0.91 per millimeter increase; 95% CI, 0.82–0.99) and reduced leukoaraiosis (OR, 5.79; 95% CI, 1.40–29.61) were independently related to good collateral status.
CONCLUSIONS: Among patients with embolic large-vessel occlusion, reduced leukoaraiosis, noncardiac embolism mechanisms including embolisms of arterial or undetermined origin, and shorter thrombus length in cardioembolism are indicators of good collateral flow.
ABBREVIATIONS:
- LCS
- leptomeningeal collateral status
- LVO
- large-vessel occlusion
Patients with ischemic stroke caused by the large-vessel occlusions (LVOs) of embolic origin often have large initial hypoperfusion lesions, extensive subsequent infarction, and poor long-term functional outcomes.1 To maintain perfusion to penumbral tissue in acute stroke caused by LVO, acute leptomeningeal collateral flow is vital. Leptomeningeal collaterals are pre-existing, small arteriolar anastomoses that cross-connect the distal-most arterioles within the crowns of the cerebral arterial trees and provide an alternative route for blood flow to fields supplied by the occluded artery, thus reducing hypoperfusion and infarct extent.2
The robustness of acute leptomeningeal collateral flow varies widely among patients with LVO ischemic stroke. However, the frequency, determinants, and outcomes of this variability have been incompletely characterized.3 Genetic factors have been suggested as an important determinant of collateral flow in preclinical rodent stroke models4 but have not been extensively studied in human patients. Some premorbid vascular risk profiles such as older age, prior hypertension, and metabolic syndrome have been associated with worse leptomeningeal collateral flow, but findings have been contradictory.2,3,5⇓⇓-8 Recently, it has also been reported that increasing severity of leukoaraiosis, which is thought to originate from chronic arteriolosclerosis and chronically reduced deep cerebral blood flow, is associated with poor leptomeningeal collateral flow.9,10 Additionally, factors present at the time of stroke onset have been reported to be associated with collateral flow status, including stroke etiology and mechanism,1,11,12 blood pressure,5 and blood glucose levels.2,3,6 Moreover, occlusive thrombus extent and composition have been suggested to be associated with the degree of collateral robustness.13,14 Prior research has often not clearly distinguished between LVOs of embolic and local in situ atherosclerotic origin in probing associations between collateral variability and clinical factors. Further research into the potential indicators of collateral variability in embolic LVOs will improve our understanding of the collateral system and potentially identify ways to improve clinical outcomes in embolic LVOs.
CTA, which is widely used in clinical practice, offers the possibility of evaluating acute leptomeningeal collateral status by assessing the amount of vascular enhancement in the affected brain regions distal to the occluded artery.2,15 In the present study, we aimed to investigate the clinically available indicators of CTA-identified acute leptomeningeal collateral flow variability in embolic LVOs.
MATERIALS AND METHODS
Patients
All patients admitted to our institution with acute ischemic stroke who underwent acute reperfusion therapy, including thrombolysis using IV rtPA and/or endovascular reperfusion therapy, were prospectively registered in a single comprehensive stroke center database. For this study, patients were identified in this database, from January 2015 to December 2019, who met the following criteria: 1) had acute occlusion of the intracranial ICA terminus or MCA horizontal or insular segments seen on the initial CTA that was treated by endovascular reperfusion therapy; 2) had a single LVO; 3) had embolus of cardiac, artery-to-artery, other arterial, or undetermined origin as the identified stroke mechanism; 4) underwent NCCT and CTA on admission before acute reperfusion therapy, with evaluable leptomeningeal collateral status (LCS) and thrombus length; and 5) underwent MR imaging within 24 hours after admission, permitting robust evaluation of leukoaraiosis. Acute endovascular reperfusion therapy procedures were performed in accordance with national and institutional guidelines.
Patients with multiple intracranial occlusions were excluded because multiple occlusions may variably further compromise LCS and be a confounding factor. For the exclusion of patients with the intracranial in situ atherosclerotic mechanism of occlusion, patients were considered to have likely in situ occlusion on postprocedural angiograms under the following conditions: 1) a residual 50%–99% stenosis after endovascular treatment; or 2) evidence of intracranial dissection on reperfusion.
This study was overseen by the Research Ethics Committee of our institution, which approved the performance of the study without explicit research informed consent because it was a minimal-risk study involving only analysis of clinically acquired data. All study protocols and procedures were conducted in accordance with the Declaration of Helsinki. The de-identified data that support the findings of this study are available from the corresponding author on reasonable request for the purpose of replicating procedures and results.
Clinical Information
The following demographic and clinical characteristics were analyzed for each patient: sex; age; history of hypertension; history of diabetes mellitus; history of dyslipidemia; history of premorbid atrial fibrillation; history of smoking; history of stroke/TIA; premorbid antithrombotic medication; premorbid statin medication; body mass index; NIHSS score on emergency admission; initial systolic/diastolic blood pressure; initial glucose, Cr, and blood urea nitrogen blood levels; stroke subtype; time from last known well to imaging; occluded target vessel; and ASPECTS from admission NCCT. The stroke subtypes were determined by expert stroke neurologists in our institution using the ASCOD (A, atherosclerosis; S, small-vessel disease; C, cardiac pathology; O, other causes; and D, dissection) phenotyping scheme, including cardioembolic stroke, artery-to-artery embolic stroke secondary to extracranial carotid arterial stenosis of >50% due to atherosclerosis, artery-to-artery embolic stroke secondary to extracranial dissection, and embolic stroke of undetermined source. The degree of carotid artery stenosis was confirmed by DSA during the thrombectomy procedure using the NASCET criteria. The time of imaging was defined as the time point at which the first images were acquired for the patient after admission. The clinical outcome of each patient, measured by the mRS score at 3 months after admission, was also collected. Favorable outcome was defined as an mRS score of 0–2 at 3 months.
Imaging Protocols
Institutional NCCT and CTA scans were performed on emergency admission using Somatom Sensation 64-detector series scanners (Siemens). First, NCCT images were obtained sequentially with the following parameters: FOV, 230 mm; voltage, 120 kV; effective current, 250–350 mAs; section collimation, 1.2 mm; effective section thickness, 3 mm; and reconstruction kernels, H40s medium for brain window and H60 for bone window. Next, CTA was performed with the nonionic monomer iohexol (Omnipaque; GE Healthcare) at 350-mg iodine/mL. Contrast-enhanced image acquisition was performed after a single-bolus IV injection of 150 mL of contrast medium at a rate of 3–5 mL/s, autotriggered by the appearance of contrast in an ROI manually placed in the ascending aorta. The vessels were scanned in helical mode with the following parameters: tube voltage, 120 kV; tube current, 300 mAs; collimation, 0.6 mm; and effective section thickness, 1 mm. CTA was performed immediately after the acquisition of NCCT images. In addition, the delayed-phase contrast-enhanced image acquisition (80 seconds post-CTA contrast injection) was obtained using the same parameters used for CTA in accordance with our institution’s stroke code protocol to reduce vascular nonopacification and increase the frequency of the identification of the proximal and distal ends of the occlusive thrombus.16 In cases in which IV rtPA was administered, patients were treated after the acquisition of delayed-phase contrast-enhanced images.
MR imaging was performed within 24 hours after admission on 1.5T Magnetom Avanto or 3T Magnetom Trio MR imaging scanners (Siemens). The standard MR imaging protocol included DWI (TR, 5600 ms; TE, 106 ms; FOV, 255 mm; matrix, 192 × 192; section thickness, 5 mm); FLAIR (TR, 9000 ms; TE, 89 ms; FOV, 220 mm; matrix, 320 × 216; section thickness, 5 mm); and MRA of the cervical and intracranial vessels.
Imaging Analyses
Imaging data for all patients were independently reviewed by 2 experienced stroke neurologists. Imaging analyses were performed without any knowledge of the clinical findings except for the side of the stroke lesion.
LCS was assessed on 20-mm CTA MIP images using a scale of 0–3:15,17 0 = absent collateral supply to the occluded vascular territory; 1 = collateral supply filling ≤50% but >0% of the occluded vascular territory; 2 = supply filling >50% but <100% of the occluded vascular territory; and 3 = 100% collateral supply of the occluded vascular territory. Rater discrepancies were settled by consensus discussions. Good LCS was defined as collateral scores of 2–3.2,3
Leukoaraiosis was assessed using FLAIR, and the Fazekas scale was used to categorize the degree of leukoaraiosis. This scale divides the WM into periventricular and deep regions, and each region is given a grade from 0 to 3, depending on the size and confluence of lesions on MR imaging; a higher score indicates a greater degree of leukoaraiosis. The examinations were scored in unaffected hemispheres of the present stroke using all available slices, rather than the single supraganglionic and single ganglionic slices from the original methodology.10 Rater discrepancies were settled by consensus discussions. The total Fazekas score was obtained as a grade from 0 to 6 by summing scores from the periventricular and deep WM lesions.
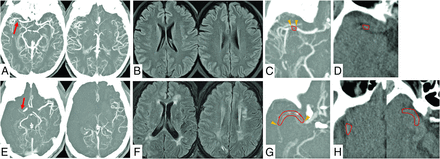
For the occlusive thrombus signals on imaging, we assessed thrombus length and thrombus density on CT scans. Horos, Version 3.3.5, software (http://www.horosproject.org) was used to reconstruct 2D MPR images in the axial, coronal, and sagittal planes and measure quantitative values of length and density on DICOM images. First, the site of occlusive thrombus was identified on CTA together with the delayed-phase images. The proximal and distal ends of the thrombus were identified by the filling defect of contrast on delayed-phase images, and thrombus length was measured using a tracer tool that traced the profile of the whole thrombus within the arterial tree in the axial, coronal, and sagittal planes.13 For thrombus involving the bifurcation, only the longest branch was used for the measurement. The longest length on any of these planes was set as the thrombus length. Next, the proximal and distal ends of the thrombus were identified in the axial plane on NCCT under the guidance of the filling defect of contrast on delayed-phase images. As support for the detection of occlusive thrombus, CTA and delayed-phase images were displayed simultaneously with the NCCT images. ROIs were manually placed on the whole thrombus on NCCT images, and the Hounsfield unit density of the thrombus was then measured by averaging all of the voxels within the ROIs. If ROIs were placed on multiple slices, the mean value (calculated by averaging all voxels within all ROIs) was used as the Hounsfield unit density of the thrombus. For the length and density of each thrombus, the results of the 2 raters were averaged. Representative cases are shown in Fig 1.
Exemplary cases of an imaging assessment with good (A–D) and poor (E–H) acute leptomeningeal collateral flow. A, CTA shows right proximal MCA occlusion (arrow) with a collateral score of 3. B, MR imaging shows mild leukoaraiosis with a total Fazekas score of 1. C, Delayed-phase CTA shows a short occlusive thrombus identified by the filling defect of contrast (between arrowheads, with a tracer tool for measuring thrombus length). D, NCCT shows an occlusive thrombus with the placement of a single ROI for measuring Hounsfield unit density (outlined region). E, CTA shows right proximal MCA occlusion (arrow) with a collateral score of zero. F, MR imaging shows severe leukoaraiosis with a total Fazekas score of 5. G, Delayed-phase CTA shows a long occlusive thrombus identified by the filling defect of contrast (between arrowheads, with a tracer tool for measuring thrombus length). H, NCCT shows an occlusive thrombus on multiple slices, with the placement of 2 ROIs for measuring Hounsfield unit density (outlined regions).
Histopathologic Analyses of Retrieved Thrombi
We investigated the histopathologic characteristics of the occlusive thrombi using specimens retrieved by thrombectomy, as reported in Liebeskind et al.18 Fragmented pieces of retrieved thrombi were collected and fixed in a 10% phosphate-buffered formalin solution for 1 day. Formalin-fixed specimens were embedded in paraffin, cut at 8-μm thickness, and stained with H&E. Histopathologic evaluation was performed without any knowledge of the clinical and radiographic findings. Stained slides were scanned at ×400 magnification using an Aperio ScanScope XT digital scanner (Aperio). ImageJ software (National Institutes of Health) was used to quantify the percentages of red blood cells, white blood cells, and fibrin by area. These measurements were repeated for each fragment of thrombus retrieved from the entire procedure. When multiple fragments were retrieved for analysis, the mean values across fragments were used for each constituent.
Statistical Analyses
Statistical analyses were performed using JMP 13.0 software (SAS Institute). Categoric data are expressed as numbers (percentages), and continuous data are expressed as medians (interquartile ranges). Patients’ clinical and radiographic characteristics and the histopathologic characteristics of the thrombi were compared between the good LCS group (collateral scores of 2–3) and the poor LCS group (collateral scores of 0–1). For comparisons between 2 groups of patients, variables were analyzed using the Fisher exact test or Pearson χ2 test for categoric data and the Mann-Whitney U test for continuous data, as appropriate. In the analyses, stroke subtypes were dichotomized into cardioembolic stroke and other noncardiac embolic stroke subtypes. The total Fazekas score was further examined by receiver operating characteristic curve analysis, and the cutoff value related to good LCS was calculated. A multivariate logistic regression analysis model, adjusted for sex, age, and variables with P < .05 in the univariate analysis, was built to assess the influence of clinical factors on good LCS. Subgroup analyses on the group of patients with cardioembolic stroke were performed in the same manner. Interrater reliability for the data before resolving disagreements, as measured by a quadratic weighted Cohen κ, was excellent, with κ = 0.87 for LCS and κ = 0.81 for the Fazekas score. For all statistical analyses, values of P < .05 were considered significant.
RESULTS
Patient Characteristics
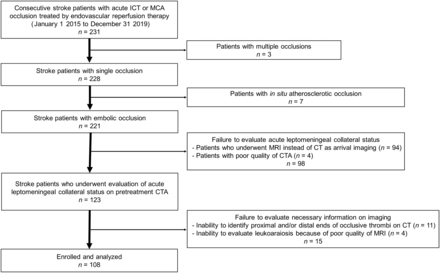
Among 231 consecutive patients with acute ischemic stroke caused by ICA terminus or MCA occlusion treated by endovascular reperfusion therapy from January 1, 2015, to December 31, 2019, exclusions were for multiple occlusions (3 patients), in situ atherosclerotic occlusion (7 patients), initial MR imaging instead of CT as arrival imaging (94 patients), poor quality of CTA (4 patients), inability to visualize the full extent of thrombus (11 patients), and poor quality of MR imaging within 24 hours (4 patients) (Fig 2). Among the 108 patients fully meeting the study inclusion criteria, 49 patients (45%) were women, the median age was 76.5 years (interquartile range, 63.3-86 years), and the median NIHSS score on admission was 18 (interquartile range, 11.25–22) (detailed characteristics are listed in the Online Supplemental Data). The occluded vessels were the ICA terminus in 33 patients (31%), the horizontal segment of the MCA in 57 patients (53%), and the insular segment of the MCA in 18 patients (17%). Embolic LVO stroke subtype was most often cardioembolic (82 patients [76%]), followed by atheroembolic stroke due to extracranial carotid artery atherosclerotic stenosis (12 patients [11%]). The median time from last known well to imaging was 104 minutes (interquartile range, 62–379 minutes).
Flow diagram of the patients screened, enrolled, and analyzed in the study. ICT indicates internal carotid artery terminus.
Clinical Indicators of Acute Leptomeningeal Collateral Flow
Good LCS (collateral scores of 2–3) was present in 67 patients (62%) (zero, 8 patients [7%]; one, 33 patients [31%]; two, 51 patients [47%]; and three, 16 patients [15%]). Demographic and clinical characteristics of the good and poor LCS groups are shown in the Online Supplemental Data. Patients with good LCS had lower presenting NIHSS scores (15 [10–22] versus 19 [16–24.5]; P = .001), a lower incidence of cardioembolic stroke (69% versus 89%; P = .04), a lower total Fazekas score (2 [1–3] versus 4 [3–5]; P < .001), and shorter occlusive thrombus length (9.8 [6.7–15.6] versus 14.7 [8.7–20.2] mm; P = .01). There were no significant differences between the 2 groups in thrombus Hounsfield unit densities or thrombus histopathologic compositions.
In the receiver operating characteristic curve analysis, the optimal cutoff value for the total Fazekas score related to good LCS was 2 (area under the curve, 0.81; P < .001; sensitivity, 0.68; specificity, 0.83) (total Fazekas scores of 0–2, thirty-nine patients [36%]; and 3–6, sixty-nine patients [64%]). Of the 39 patients with total Fazekas scores of 0–2, LCS was poor in only 5 patients.
In the multivariate logistic regression analysis identifying factors independently associated with good LCS, cardioembolic stroke subtype was inversely related to good LCS (OR, 0.17; 95% CI, 0.02–0.87; P = .03), and reduced leukoaraiosis (total Fazekas scores, 0–2) was positively related to good LCS (OR, 9.57; 95% CI, 2.49–47.75; P < .001) (Table 1). Favorable clinical outcomes were achieved more frequently in patients with good compared with poor LCS (49% versus 16%; P = .006).
Clinical indicators independently associated with good acute leptomeningeal collateral flow on multivariate analysis
Subgroup Analyses of Patients with Cardioembolic Stroke
Of the 82 patients with cardioembolic stroke, 46 patients (56%) had good LCS (zero, 8 patients [10%]; one, 28 patients [34%]; two, 34 patients [41%]; and three, 12 patients [15%]). Compared with other patients with noncardiac embolic stroke, patients with cardioembolic stroke were older (79.5 [68.3–89.3] versus 65 [55–75] years; P < .001), had higher total Fazekas scores (3 [2–4] versus 2 [1–3]; P = .03), and had, less commonly, a good LCS (56% versus 81%; P = .04) (Online Supplemental Data).
Patients with cardioembolic stroke with good LCS compared with those with poor LCS had lower presenting NIHSS scores (15 [9–22] versus 19.5 [16.25–25]; P = .002), lower blood glucose levels (6.42 [5.83–7.39] versus 7.28 [6.26–8.26] mmol/L; P = .02), lower total Fazekas scores (2 [1–3.25] versus 4 [3–5]; P < .001), and shorter occlusive thrombus length (10.7 [7.2–14.3] versus 16.6 [9.6–20.5] mm; P = .007) (Online Supplemental Data). In multivariate logistic regression analysis, shorter thrombus length (OR, 0.91 per millimeter increase; 95% CI, 0.82–0.99; P = .04), and reduced leukoaraiosis (OR, 5.79; 95% CI, 1.40–29.61; P = .01) were independently related to good LCS (Table 2).
Clinical indicators independently associated with good acute leptomeningeal collateral flow in patients with cardioembolic stroke on multivariate analysis
DISCUSSION
In the present study, among patients with acute ischemic stroke caused by LVOs due to embolism, good collateral robustness was present in >6 of every 10 patients. Independent indicators of good collateral flow were reduced leukoaraiosis, noncardiac embolism mechanism including embolism of arterial or undetermined origin rather than cardiac embolism, and occlusive thrombi of substantially shorter length. In addition, in patients with good collateral flow, neurologic deficits on presentation were less severe, and independent functional outcomes at 3 months were achieved 3 times more often.
Of the chronic factors in patients’ premorbid vascular risk profiles, reduced leukoaraiosis was positively associated with good leptomeningeal collateral flow in embolic LVO. Our finding is in agreement with those in previous studies.9,10 Leukoaraiosis is thought to originate from small-vessel disease, which is caused by chronically reduced CBF due to arteriolosclerosis, lipohyalinosis, and fibrinoid necrosis of the small arteries and arterioles.19 The leptomeningeal collateral system is also composed of small arteries and arterioles. Therefore, it is possible that mechanisms causing leukoaraiosis might also affect the arterioles of the leptomeningeal collateral system.10 On the other hand, 1 study reported that leukoaraiosis had no relationship with the extent of collaterals.20 Our study differed from that study in an important aspect. Our cohort contained appropriate numbers of patients with severe leukoaraiosis, while almost all the patients had absent or minimal leukoaraiosis in the previous study. In contrast, age and vascular risk factors including a history of hypertension, diabetes mellitus, dyslipidemia, smoking, and obesity were not univariately associated with poor leptomeningeal collateral flow in the present study, though the severity of leukoaraiosis has been reported to be associated with several of these parameters, including older age, hypertension, diabetes mellitus, smoking, and obesity.21,22 Our results indicate that leukoaraiosis, as a physiologic expression of cumulative small-vessel injury from multiple risk factors, more strongly correlates with leptomeningeal impairment. Although leptomeningeal impairment may also arise, in part, from the joint effect of multiple vascular risk factors, the chronic small-vessel injury of leukoaraiosis itself and its associated chronic mild deep ischemic stress may be an important contributor to impairing pial collateral recruitment. Leukoaraiosis might, therefore, be an imaging marker of global cerebrovascular dysfunction and a determinant of leptomeningeal collateral variability.
Among the acute factors present at the time of stroke, cardioembolic stroke subtype, rather than other noncardiac embolic stroke subtypes including embolism of arterial or undetermined origin, was associated with poor leptomeningeal collateral flow. Similar findings have been demonstrated in several prior studies.1,11,12 However, our study complements the prior studies by having excluded intracranial atherosclerotic disease and comparing cardioembolic stroke only to other noncardiac embolic strokes, most often of extracranial atherosclerotic origin. Atheroembolic stroke is preceded by arterial stenosis that develops across decades, which may promote cerebral collateral circulation because of the chronic and slowly progressive nature of cerebral large-vessel hypoperfusion.23 In contrast, cardioembolic stroke usually has an abrupt onset without chronic cerebral large-vessel hypoperfusion, which may be associated with a paucity of collateral artery formation and recruitment.24 In addition, compared with patients with other noncardiac embolic strokes, patients with cardioembolic stroke were older and had higher total Fazekas scores, which could also impair pial collateral recruitment caused by small-vessel injury. Thus, the underlying stroke etiology may be a determinant of leptomeningeal collateral flow.
Shorter thrombus length was another acute factor associated with good leptomeningeal collateral flow in cardioembolic stroke. Few studies have assessed the relationship between occlusive thrombus characteristics and collateral flow.13,14 Theoretically, a longer thrombus might obstruct more orifices of arteries that provide collateral blood flow, leading to decreased pial collateral recruitment to the occluded vascular territory.14 Conversely, collaterals may possibly influence thrombus length, and longer thrombus length may result causally from poor collateral flow because patients with poor collaterals have an increased stasis around the occlusive thrombus, leading to thrombus extension.13 Previous studies have suggested that shorter thrombus length may be a consequence rather than a cause of good collateral flow.13 However, further studies using time-series data are necessary to analyze the causal relationships. On the other hand, we did not identify any relationship between leptomeningeal collateral flow and the histopathologic components of the whole occlusive thrombus. In future studies investigating the relationship between collateral circulation and the histopathologic components of occlusive thrombus, we, therefore, suggest that occlusive thrombi should be analyzed, if possible, in a manner that differentially assesses the original thrombus component (which comes from a proximal source) and the new thrombus component (which forms in situ from the stasis of blood flow around the original thrombus).13
Good leptomeningeal collateral flow after embolic occlusion was associated with lower NIHSS scores at presentation and favorable functional outcomes at 3 months. Previous studies have also reported that more robust CTA collaterals were predicted by lower NIHSS scores and were associated with better clinical outcomes.25,26 Poorer leptomeningeal collateral flow has been reported to be associated with both larger initial infarction size and larger follow-up infarct progression volumes, leading to poorer functional outcomes and increased mortality.25 In addition, poorer collaterals may serve as an indicator of a worse response to reperfusion therapy and higher rates of intracranial hemorrhage after reperfusion therapy, which may partly explain the unfavorable effects of poor collaterals.27 The findings that patients with good collaterals showed favorable outcomes stress the need to clarify the clinically useful indicators of good leptomeningeal collateral variability, to provide appropriate acute stroke treatments and improve clinical outcomes in embolic LVOs. Premorbid leukoaraiosis, underlying stroke etiology, and occlusive thrombus length may be helpful indicators of acute leptomeningeal collateral flow in clinical practice.
Some limitations of our study should be noted. This was a single-center study, and replication in other populations is desirable to demonstrate generalizability. The power to detect clinical features with less marked effects on collateral flow robustness may have been constrained by sample size, despite the relatively large, well-studied population examined in our study. In addition, the multivariate analysis model was built using variables with P < .05 in the univariate analysis due to the modest sample size, and some potentially relevant factors, such as an occluded vessel site, might have been excluded from the model. All our patients underwent the assessment of LCS using single-phase CTA, which may have led to the underestimation of LCS in the case of delayed filling in combination with an early acquisition phase.28 Newer techniques, such as CTA reconstruction from CTP data and multiphase CTA, make CTA independent of the timing of contrast administration and scan acquisition and may improve the assessment of LCS.29,30
The criterion standard for assessing LCS is DSA.8 However, multivessel complete DSA is not always performed, and we aimed to determine features associated with collateral extent on presentation. Although the Fazekas scale was useful to evaluate the degree of leukoaraiosis, the scale is relatively crude. Measuring quantitative volumes of WM lesions may allow more accurate evaluation of leukoaraiosis. While noncardiac rather than cardiac embolism was associated with good collateral flow, the noncardiac embolic stroke subgroup might have potentially contained a different extent of collaterals according to different stroke etiologies. We did not analyze the circle of Willis for anatomic variants, possibly a confounding factor.2
CONCLUSIONS
Among patients with embolic LVOs, good collateral flow is present in 62% of patients and is associated with better clinical outcomes. Reduced leukoaraiosis, a noncardiac embolism mechanism including embolism of arterial or undetermined origin rather than a cardiac embolism mechanism, and shorter thrombus length in cardioembolism are indicators of good collateral flow. These findings suggest that chronic small-vessel injury as well as an abrupt stroke onset without chronic large-vessel hypoperfusion may reduce collateral robustness, and they also suggest that improvement of collaterals may protect against thrombus extension.
Footnotes
This work was supported by the Uehara Memorial Foundation (grant No. 201840008), the Fukuda Foundation for Medical Technology (Research Training Award), and the Japanese Society of Neurology (Overseas Training Program for Young Researchers).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received May 20, 2021.
- Accepted after revision September 20, 2021.
- © 2022 by American Journal of Neuroradiology