Abstract
BACKGROUND AND PURPOSE: Whole-brain network connectivity has been shown to be a useful biomarker of cerebral amyloid angiopathy and related cognitive impairment. We evaluated an automated DTI-based method, peak width of skeletonized mean diffusivity, in cerebral amyloid angiopathy, together with its association with conventional MRI markers and cognitive functions.
MATERIALS AND METHODS: We included 24 subjects (mean age, 74.7 [SD, 6.0] years) with probable cerebral amyloid angiopathy and mild cognitive impairment and 62 patients with MCI not attributable to cerebral amyloid angiopathy (non-cerebral amyloid angiopathy–mild cognitive impairment). We compared peak width of skeletonized mean diffusivity between subjects with cerebral amyloid angiopathy–mild cognitive impairment and non-cerebral amyloid angiopathy–mild cognitive impairment and explored its associations with cognitive functions and conventional markers of cerebral small-vessel disease, using linear regression models.
RESULTS: Subjects with Cerebral amyloid angiopathy–mild cognitive impairment showed increased peak width of skeletonized mean diffusivity in comparison to those with non-cerebral amyloid angiopathy–mild cognitive impairment (P < .001). Peak width of skeletonized mean diffusivity values were correlated with the volume of white matter hyperintensities in both groups. Higher peak width of skeletonized mean diffusivity was associated with worse performance in processing speed among patients with cerebral amyloid angiopathy, after adjusting for other MRI markers of cerebral small vessel disease. The peak width of skeletonized mean diffusivity did not correlate with cognitive functions among those with non-cerebral amyloid angiopathy–mild cognitive impairment.
CONCLUSIONS: Peak width of skeletonized mean diffusivity is altered in cerebral amyloid angiopathy and is associated with performance in processing speed. This DTI-based method may reflect the degree of white matter structural disruption in cerebral amyloid angiopathy and could be a useful biomarker for cognition in this population.
ABBREVIATIONS:
- CAA
- cerebral amyloid angiopathy
- CMB
- cerebral microbleeds
- CSO-PVS
- perivascular spaces in the centrum semiovale
- cSS
- cortical superficial siderosis
- cSVD
- cerebral small vessel disease
- ICH
- intracerebral hemorrhage
- IQR
- interquartile range
- MCI
- mild cognitive impairment
- MD
- mean diffusivity
- MMSE
- Mini-Mental State Examination
- PSMD
- peak width of skeletonized mean diffusivity
- nTBV
- normalized total brain volume
- nWMHV
- normalized white matter hyperintensity volume
- WMH
- white matter hyperintensities
Sporadic cerebral amyloid angiopathy (CAA) is a highly prevalent cerebral small-vessel disease (cSVD) in the elderly.1 CAA is a well-known cause of lobar intracerebral hemorrhage (ICH) and is also increasingly recognized as a major contributor to vascular cognitive impairment and dementia.2,3 Although underlying mechanisms leading to cognitive impairment in CAA remain uncertain, it has been hypothesized that recurrent vascular lesions cause progressive disruption of the brain's structural connectivity, compromising network efficiency.4,5 Conventional MR imaging markers of CAA, including lobar cerebral microbleeds (CMB),6 cortical superficial siderosis (cSS),7 white matter hyperintensities (WMH),8 and cortical microinfarcts 9 have been linked to cognitive functions. However, these associations are mostly weak and inconsistent across studies, suggesting that these markers may reflect only the tip of the iceberg in the whole spectrum of vascular pathology.10
Accumulating evidence suggests that DTI methods detect loss of microstructural integrity and other abnormalities not captured by structural MRI and tend to show stronger associations with cognition in subjects with cSVD.11,12 Yet, the direct application of DTI in routine clinical practice is hampered by highly variable, complex, and time-consuming processing techniques.
Peak width of skeletonized mean diffusivity (PSMD) is a recently developed, fully automated DTI marker based on the skeletonization of white matter tracts and histogram analysis of mean diffusivity (MD).13 PSMD has been shown to be particularly sensitive to vascular-related white matter abnormalities, demonstrating consistent associations with processing speed in subjects with cSVD.13 However, despite the common nature and high prevalence of CAA in aging populations, potential applications of PSMD in CAA have been scarcely investigated.
In the current study, we tested whether PSMD reflects the burden of underlying cSVD and cognitive dysfunctions in subjects with CAA. Among subjects with mild cognitive impairment (MCI) recruited specifically from a memory clinic setting, we explored the following: 1) whether PSMD is increased in subjects with CAA compared with those with non-CAA, 2) whether it is associated with structural MRI markers of CAA, and 3) whether it is correlated with cognitive functions.
MATERIALS AND METHODS
The data supporting findings of this study are available from the corresponding author on reasonable request.
Participants
We analyzed data from a memory clinic research cohort from the Massachusetts General Hospital between March 2010 and October 2016 and designed a case-control study. Patients underwent clinical examination, neuropsychological evaluation, and research MRI. The Institutional Review Board of Massachusetts General Hospital approved this study, and written informed consent was obtained from all participants or their surrogates.
We included subjects 55 years of age or older meeting Petersen criteria (2004)14 for MCI based on clinical assessment of functional status, neurologic evaluation, and extensive neuropsychological assessment. On visual examination of research MRIs, patients with MCI were categorized as the following: 1) CAA-MCI if they fulfilled the Modified Boston criteria15 for probable CAA (55 years of age or older; multiple lobar CMB with or without cSS or a single lobar cerebral microbleed and the presence of cSS), or 2) non-CAA-MCI. In both groups, exclusion criteria were dementia, history of symptomatic or asymptomatic ICH (defined as hemorrhagic focus of > 5 mm in diameter), the presence of deep CMB (suggesting arteriolosclerosis as underlying cSVD), contraindications for MRI, and the presence of excessive motion artifacts on DTI on careful qualitative visual inspection.
Data Collection
We systematically collected demographic information and medical history for each participant. All subjects underwent a standardized neuropsychological test battery, as previously described.9 Global cognitive status was assessed with the Mini-Mental State Examination (MMSE).16 Performance on neuropsychological tests was clustered to create composite scores exploring specific cognitive domains:17executive function (Trail-Making Test B18 and Digit Span Backward19), processing speed/attention (Trail-Making Test A,20 Digit Span Forward, and the Wechsler Adult Intelligence Scale, Third Edition Digit Symbol Coding19), memory (Hopkins Verbal Learning Test–Revised21 and Wechsler Memory Scale for logical memory,22 immediate recall, and delayed recall), and language function (Boston Naming Test23 and Animal Naming Test24). Performance on each test was first transformed into sex-, age-, and education-adjusted z scores using published normative data.16,24⇓-26 Then, the z scores were averaged within each composite domain to obtain domain-specific scores for each subject.
MRI Acquisition
Neuroimaging was acquired on a 3T MRI scanner (TIM Trio; Siemens), using a 32-channel head coil. MRI sequences included high-resolution diffusion-weighted imaging (60 directions; TR = 8040 ms; TE = 84 ms; slice thickness = 2 mm; in-plane resolution = 2 × 2 mm; b-value = 700 s/mm2), 3D T1-weighted multiecho (TR = 2300 ms; TE = 2.98 ms; slice thickness = 1 mm; in-plane resolution = 1 × 1 mm), 3D FLAIR (TR = 6000 ms; TE = 455 ms; slice thickness = 1 mm; in-plane resolution = 1 × 1 mm), and SWI (TR = 27 ms; TE = 20 ms; slice thickness = 1.5 mm; in-plane resolution = 0.86 × 0.86 mm).
The median delay between neuropsychological evaluation and MRI was 1.85 months (interquartile range [IQR] = 0.00–3.06 months) and was shorter in subjects with CAA-MCI compared with those with non-CAA-MCI (median, 0 [IQR = 0.0–0.24] months versus 2.1 [1.17–3.30] months; P < .001).
DTI and PSMD Processing
PSMD was calculated from unprocessed DTI data using a publicly available script (PSMD Marker, Version1.0; http://www.psmd-marker.com).13 This fully automated pipeline relies on the FMRIB Software Library (FSL, Version 6.0.1; http://www.fmrib.ox.ac.uk/fsl) for the preprocessing of DTI data (eddy current and motion correction, [eddy_correct https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/eddy]), brain extraction (FSL Brain Extraction tool; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET), and tensor fitting (dtifit; http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/fdt/fdt_dtifit.html), followed by skeletonization of preprocessed DTI data, application of a custom mask, and histogram analysis (Fig 1). Precisely, DTI data were skeletonized using the Tract-Based Spatial Statistics procedure (TBSS; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS), part of the FSL, and the FMRIB 1-mm fractional anisotropy template (FA template; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases, thresholded at a fractional anisotropy value of 0.2). MD images were projected onto the skeleton, using the fractional anisotropy–derived projection parameters. Next, to avoid contamination of the skeleton through CSF partial volume effects, we further masked the MD skeleton with a standard skeleton thresholded at a fractional anisotropy value of 0.3 and a mask provided with the PSMD pipeline to exclude regions adjacent to the ventricles, such as the fornix. Finally, PSMD was calculated as the difference between the 95th and fifth percentiles of the MD values of voxels contained within the skeleton.13 To ensure that results were not driven by outliers, we identified extreme PSMD (values below 1.5 × IQR from the first quartile or above 1.5 × IQR from the third quartile) and excluded them from analyses.
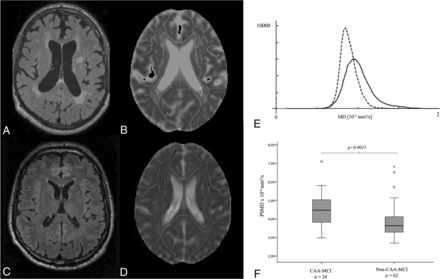
FLAIR images from a subject with CAA-MCI (A) and one with a non-CAA-MCI (B), demonstrating different burdens of WMH. MD maps display the skeletonized WM tracts from the same subjects with CAA-MCI (C) and non-CAA-MCI (D). E, Histograms depict the MD values of the voxels contained in the WM tract skeleton from the same subjects with CAA-MCI (solid line) and non-CAA-MCI (dashed line). F, The boxplot represents group differences in PSMD between CAA-MCI and non-CAA-MCI. The dagger indicates the results derived from ANCOVA, adjusting for age at MRI (P < .001).
Neuroimaging Markers of cSVD
MRI markers of cSVD were quantified by investigators blinded to all clinical data and according to the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) recommendations.27
The presence, number, and location of CMB were evaluated on the SWI according to the current consensus criteria.27,28 They were classified as lobar when located in cortical and corticosubcortical areas. cSS was visually assessed according to recently proposed criteria and transformed into a dichotomous variable (absence versus presence).29 Perivascular spaces in the centrum semiovale (CSO-PVS) were rated on axial T1-weighted MRI, according to a previously developed score,30 and were analyzed as both dichotomous (≤2 versus >2) and ordinal variables. CMB, cSS, and CSO-PVS were analyzed by 2 experienced raters (N.R. and D.S.) using validated scales, and final ratings were obtained via a consensus.
After visual inspection of MR image quality, WMH volume, total brain volume, and total intracranial volume were calculated using FreeSurfer, Version 5.3 (http://surfer.nmr.mgh.harvard.edu), as previously described.31 Normalized total brain volume (nTBV) was calculated as the total brain volume/intracranial volume ratio, and normalized WMH volume (nWMHV) was calculated as WMH volume/intracranial volume × 100.
Statistical Analysis
We compared the clinical and imaging characteristics between subjects with MCI with and without CAA using the χ2 or Fisher test for categoric variables and an independent t test or Mann-Whitney U test for continuous variables, as appropriate. The distribution of continuous variables was tested for normality with the Shapiro-Wilk test.
Log-transformed PSMD values were compared between patients with CAA-MCI and those with non-CAA-MCI using ANOVA, adjusted for age. We further adjusted for log-transformed nWMHV and cognitive status (MMSE).
Receiver operating characteristic curve analyses were used to quantify the performance of PSMD and nWMHV in discriminating subjects with CAA from those with non-CAA.
The association between PSMD and MRI markers of cSVD and PSMD and cognitive performances was evaluated in CAA-MCI and non-CAA-MCI separately. Linear regression models were used to explore relationships between PSMD and structural MRI markers of cSVD (lobar CMB count, CSO-PVS score, the presence of cSS, nWMHV, and nTBV), adjusting for age. The association between PSMD and cognitive scores in each domain was explored using linear regression models in both groups separately, adjusting for structural MRI markers of cSVD and the time delay between the neuropsychological evaluation and the MRI. Because cognitive scores were already adjusted for age, sex, and education level, these variables were not included in the models.
The statistical significance level was set at .05 for all analyses. We used the Statistical Package for the Social Sciences, Version 24.0 for Windows (IBM) and R (v3.5.3) for statistical analysis.
RESULTS
We identified 134 subjects with cognitive impairment enrolled in this prospective study who underwent a research MRI. Of them, 42 subjects were excluded on the basis of the prespecified criteria: diagnosis of dementia (n = 6), possible CAA category (n = 10), presence of deep CMB (n = 5), lack of neuropsychological tests (n = 19), and the presence of excessive motion artifacts on DTI, based on qualitative visual inspection (n = 2). Additionally, 3 outliers with extreme PSMD values (all with high values) were identified in each group and were excluded from the analysis. The final cohort consisted of 86 subjects with MCI (mean age, 73.7 [SD, 7.7] years; 38.4% female) without a history of ischemic stroke or ICH, including 24 subjects with probable CAA (CAA-MCI; 27.9%) and 62 without CAA (non-CAA-MCI; 72.1%).
Comparison between Subjects with CAA and Those with Non-CAA
Subjects with CAA-MCI and non-CAA-MCI were similar in age and vascular risk factors (Table 1). MMSE scores were lower in subjects with CAA-MCI compared with subjects with non-CAA-MCI (P = .003). Patients with CAA-MCI had worse performance in memory than subjects with non-CAA-MCI (P = .005). The 2 groups had similar scores across all other cognitive domains (P > .05, for all). Compared with those with non-CAA-MCI, subjects with CAA-MCI presented with a higher burden of MRI markers of cSVD, including a higher prevalence of cSS (P < .001), higher lobar CMB count (P < .001), greater nWMHV (P = .016), a higher prevalence of high CSO-PVS scores (P < .001), and lower nTBV (P = .004). PSMD values were significantly higher in CAA-MCI in comparison with non-CAA-MCI (P < .001) (Fig 1F), even after adjusting for age (P < .001). In a post hoc analysis, we found that PSMD remained significantly higher in subjects with CAA-MCI compared with subjects with non-CAA-MCI when further controlling for nWMHV (P = .007) or cognitive status (MMSE z scores) (P < .001). In receiver operating characteristic analyses, PSMD (area under the curve = 0.755; 95% CI, 0.636–0.873; P < .001) was able to significantly discriminate subjects with CAA from those without CAA, and it yielded a greater area under the curve than nWMHV (area under the curve = 0.668; 95% CI, 0.544–0.792; P = .016) (Online Supplemental Data).
Baseline characteristics of subjects with CAA-MCI and those with non-CAA-MCI
Associations between PSMD and Markers of cSVD
In linear regression analyses adjusted for age, increased PSMD was associated with greater nWMHV both in CAA-MCI (β = 0.75; P < .001) and non-CAA-MCI (β = 0.69; P < .001) groups, but not with nTBV, CMB, CSO-PVS, or cSS (Table 2). In multiple regression models including all quantified structural MRI markers of cSVD, only nWMHV remained independently associated with PSMD in subjects with CAA-MCI (β = 0.66; P < .001) and non-CAA-MCI (β = 0.71; P < .001) (Table 2 and Online Supplemental Data).
Association between PSMD and MRI markers of small-vessel disease in subjects with CAA-MCI and non-CAA-MCIa
Associations between PSMD and Cognitive Function
In the CAA-MCI group, multiple regression models accounting for lobar CMB count, cSS, CSO-PVS score, nWMHV, and nTBV demonstrated that increased PSMD was independently associated with worse performance in processing speed (β = –1.08; P = .004) (Table 3 and Online Supplemental Data). In the non-CAA-MCI group, multiple regression analyses did not reveal any significant associations between PSMD and scores reflecting each cognitive domain (Table 3 and Online Supplemental Data).
Association between PSMD and cognitive performance in subjects with CAA-MCI and non-CAA-MCIa
DISCUSSION
Several key findings emerge from this study on PSMD in patients with CAA presenting with MCI in the absence of ICH. First, subjects with MCI with CAA showed increased PSMD values compared with subjects with MCI without CAA, even after adjusting for baseline differences in age, nWMHV, and cognitive status. Second, we confirmed that PSMD was strongly associated with WMHV in our CAA population, but not with other structural markers of cSVD. Third, we found that PSMD values were associated with worse performance in processing speed among subjects with CAA-MCI after controlling for the presence of other MRI markers of cSVD. In contrast, PSMD was not associated with cognitive function in subjects with non-CAA-MCI.
PSMD studies have, so far, focused mainly on community-dwelling13,32,33 and cognitively impaired elderly subjects,13,34 as well as those with inherited13,35 and sporadic cSVD.13,36 To our knowledge, only 1 previous study has investigated the performance of PSMD in sporadic CAA, including subjects with and without ICH recruited from both stroke-prevention and memory clinics.37 Because CAA pathology is highly prevalent and significantly contributes to vascular cognitive impairment in the elderly population,38 further investigating the performance of PSMD in the context of CAA is an important step for the validation of this new neuroimaging biomarker as a surrogate for cognitive dysfunction in cSVD.39
As expected, the PSMD values we obtained in subjects with CAA in a memory clinic were remarkably similar to those found in other sporadic cSVD cohorts,13,36 including another CAA cohort,37 corroborating the reproducibility and stability of PSMD across different scanners, sequences, and even clinical samples.13,40
The observed increase in PSMD values among subjects with CAA-MCI supports the hypothesis that whole-brain microstructural integrity is impaired in this population. Our results are in accordance with previous studies showing microstructural abnormalities in CAA when relying on other DTI-based methods.11,41 Most important, PSMD offers several advantages in comparison with other DTI methods: It is a fully automated and fast technique; it offers higher interscanner reproducibility; power calculations have shown smaller sample size estimates for PSMD; and it is more strongly associated with performance in processing speed.13
Group differences in PSMD remained significant (CAA-MCI versus non-CAA-MCI) even after adjusting for age, nWMHV, and MMSE scores. This finding suggests that PSMD differences are not solely driven by these factors and may indicate that this marker, like other global DTI measures, might capture abnormalities not visible on structural MRI sequences.
In our CAA-MCI sample, PSMD was strongly associated with nWMHV, but not with hemorrhagic markers of CAA (lobar CMBs and cSS), a finding in line with those from a recent study on a different CAA sample,37 and this finding suggests that white matter tract disruption in CAA may be more closely linked to cSVD damage from ischemic rather than hemorrhagic origin.
The encouraging finding that PSMD is independently associated with processing speed in our subjects with CAA-MCI, after adjusting for other conventional MRI markers of cSVD, is in consonance with recently published results from another CAA sample.37 The lack of association between PSMD and cognition in the non-CAA-MCI sample is consistent with findings from other studies in cohorts with low burdens of cSVD.13,36 PSMD, like other DTI metrics, appears to be more sensitive to cSVD-related white matter abnormalities than to neurodegenerative pathology.13,42 The low burden of cSVD pathology observed in our non-CAA sample might explain the absence of association between PSMD and processing speed.
Our results argue in favor of a strong link between PSMD and processing speed in cSVD populations, as advocated in the original PSMD study.13 However, mechanisms underlying these strong associations with cognition are incompletely understood. McCreary et al37 reported that a greater variation in white matter MD could be seen in microarchitectural disruption caused by pathologic processes. Although the histopathologic features specifically associated with increases in PSMD remain unknown, tissue rarefaction and lower myelin density have been related to MD variations in subjects with CAA.43 It is possible that similar microstructural abnormalities underlie changes in PSMD in CAA, reflecting disruption of synaptic transmission, which could affect cognition.
Our study has limitations. The small sample size of our cohort may account for the relatively weak cognitive correlations observed. Hence, our findings should be considered preliminary and require external validation in larger CAA cohorts. By including only subjects with MCI (cognitively healthy and subjects with CAA and dementia were excluded), our study was not designed to assess relationships between PSMD and the full spectrum of cognitive impairment, ranging from MCI to dementia. Still, our significant findings in subjects with mild forms of cognitive impairment argue in favor of the robustness of PSMD as a biomarker for cognitive function in CAA. Additionally, our study included participants with a specific presentation of CAA (ie, mild cognitive symptoms without ICH). We excluded subjects with ICH because this likely represents a different phenotype of the disease.44 While we designed our study to examine this specific group of subjects with CAA who frequently present in memory clinic settings, our results cannot be generalized to other CAA populations or phenotypes. Another limitation of our study is the absence of comparisons between PSMD and other previously validated DTI-based markers to assess whether this new method constitutes a superior biomarker.
Nonetheless, this study also has several strengths and expands on previous literature by evaluating the relevance of PSMD in a specific phenotype of CAA and investigating its independent cognitive and neuroimaging associations.
CONCLUSIONS
PSMD values are higher among cognitively impaired subjects with CAA in comparison with those without CAA and are associated with nWMHV and performance in processing speed. Our preliminary results support the relevance of PSMD, a completely automated DTI-based method, in capturing microstructural brain changes in subjects with CAA, even in the absence of ICH. PSMD may serve as a biomarker in future clinical trials involving CAA and other cSVD.
Footnotes
N. Raposo and M.C. Zanon Zotin contributed equally to this work.
This study was supported by the following National Institutes of Health grants: R01AG047975, R01AG026484, P50AG005134, K23AG02872605. Nicolas Raposo was supported by a Fulbright Scholarship and received an Arthur Sachs Scholarship from the Harvard University Committee on General Scholarship and a Philippe Foundation research grant. Markus D. Schirmer was supported by the European Union's Horizon 2020 Research and Innovation Program under the Marie Sklodowska-Curie grant agreement No 753896.
Disclosures: Nicolas Raposo—RELATED: Grant: Fulbright Scholarship, Arthur Sachs Scholarship from the Harvard University Committee on General Scholarship, and a Philippe Foundation research grant; UNRELATED: Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Pfizer, Bristol Myers Squibb, Bayer. Maria Clara Zanon Zotin—RELATED: Support for Travel to Meetings for the Study or Other Purposes: Mass General Brigham, Comments: support to travel to a conference. Dorothee Schoemaker—RELATED: Grant: American Heart Association, Comments: postdoctoral fellowship.* Kristin Schwab—UNRELATED: Employment: Massachusetts General Hospital MarkVCID. Markus D. Schirmer—UNRELATED: Grants/Grants Pending: European Research Council.* Mark Etherton—UNRELATED: Grants/Grants Pending: American Academy of Neurology.* Steven Greenberg—RELATED: Grant: National Institutes of Health; UNRELATED: Consultancy: Bayer, Biogen, IQVIA, Roche, Comments: Data Safety Monitoring Boards; Grants/Grants Pending: National Institutes of Health; Royalties: UpToDate. Marco Duering—RELATED: Grant: Else Kröner-Fresenius-Stiftung*; UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Bayer Vital, Pfizer, Comments: Lecture honoraria paid to individual author. Anand Viswanathan—RELATED: Grant: National Institutes of Health*; UNRELATED: Consultancy: Biogen, Alynylam Pharmaceuticals, Roche. *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received July 17, 2020.
- Accepted after revision November 20, 2020.
- © 2021 by American Journal of Neuroradiology