Abstract
BACKGROUND AND PURPOSE: Prior studies have evaluated cochlear length using CT to select the most suitable cochlear implants and obtain patient-specific anatomy. This study aimed to test the accuracy and reliability of cochlear lateral wall length measurements using 3D MR imaging.
MATERIALS AND METHODS: Two observers measured the cochlear lateral wall length of 35 patients (21 men) with postlingual hearing loss using CT and MR imaging. The intraclass correlation coefficient (with 95% confidence intervals) was used to evaluate intraobserver and interobserver reliability for the 3D cochlear measurements.
RESULTS: The mean age of the participants was 39.85 (SD, 16.60) years. Observer 1 measured the mean lateral wall length as 41.52 (SD, 2.25) mm on CT and 41.44 (SD, 2.18) mm on MR imaging, with a mean difference of 0.08 mm (95% CI, −0.11 to 0.27 mm), while observer 2 measured the mean lateral wall length as 41.74 (SD, 2.69) mm on CT and 42.34 (SD, 2.53) mm on MR imaging, with a mean difference of −0.59 mm (95% CI, −1.00 to −0.20 mm). An intraclass correlation coefficient value of 0.90 (95% CI, 0.84−0.94) for CT and 0.69 (95% CI, 0.46−0.82) for MR imaging was obtained for the interobserver reliability for the full-turn cochlear lateral wall length.
CONCLUSIONS: CT-based 3D cochlear measurements show excellent intraobserver and interobserver reliability, while MR imaging−based lateral wall length measurements have good-to-excellent intraobserver reliability and moderate interobserver reliability. These results corroborate the use of CT for 3D cochlear measurements as a reference method and demonstrate MR imaging to be an alternative acquisition technique with comparably reliable results.
ABBREVIATIONS:
- ICC
- intraclass correlation coefficient
- LWL
- lateral wall length
Across the years, there has been an increase in the proportion of the global population with postlingual profound hearing loss, especially in developed countries.1 An effective surgical treatment for patients with profound hearing loss is a cochlear implant.2 As a result, the number of patients requiring presurgical imaging has also amplified.3,4 CT has been the reference method for evaluating bony structures and cochlear length measurements.
In the last 25 years, many studies have evaluated cochlear length using CT and conebeam CT3,4 aimed at selecting the most suitable cochlear implants and obtaining patient-specific anatomy and tonotopy.5⇓⇓⇓-9 The most common methods for cochlear length measurements include the A-value (the widest diameter of the cochlear basal turn) and measuring the cochlear circumference over 3D reconstruction.3,4 The current literature proposes that accurate measurements may help in developing custom-made cochlear implant designs per the patient’s specific anatomy and tonotopy.2 However, the radiation exposure in these CT-based measurements is a consideration; therefore, it is desirable to use alternate imaging methods like MR imaging to make these measurements.10
This study aimed to test the accuracy and reliability of MR imaging–based 3D cochlear length measurements that were previously performed by CT. A secondary aim was to test the effect of the observer’s experience on these measurements.
MATERIALS AND METHODS
This retrospective diagnostic accuracy study was conducted at the Istanbul Medeniyet University, Goztepe Prof. Dr. Suleyman Yalcin City Hospital, between January 1, 2014, and December 31, 2020. Ethics approval was obtained from the local University Ethics Committee, and the committee waived the need for informed consent due to the retrospective nature of the study. The Standards for Reporting of Diagnostic Accuracy Studies (STARD) guideline was followed during the conduct and documentation of the study.11
Study Participants
Patients with postlingual hearing loss who underwent cochlear implant surgery in our university hospital were recruited for the study based on the following inclusion and exclusion criteria.
Inclusion Criteria
Patients for whom both MRI and CT imaging were performed in our hospital
Patients whose round window niche (the cochlear outer wall and apical turn end point) could be clearly distinguished on imaging
Patients who did not have a congenital anomaly of the inner ear as seen on the CT and MRI.
Exclusion Criteria
Patients who were operated on at our hospital, but imaging was performed at another center
The image quality of the scans was inadequate for measurement
Cochlear calcifications that did not allow measurement of the lateral cochlear wall.
Protocols for CT and MR Imaging
CT was performed per the temporal bone algorithm using a Optima CT660 scanner (GE Healthcare); all patients were scanned supine. The FOV was adjusted to include the entire temporal bone, data were collected with a 512 × 512 matrix detector, and the section thickness was 0.625 mm.
MR imaging was performed using the Optima 450w 1.5T scanner (GE Healthcare). For this study, the FIESTA-C (Siemens) equivalent, the CISS sequence, was performed in the 3 in-plane acquisitions, namely axial, coronal, and oblique sagittal, through the internal auditory canal (details about this sequence are presented in Table 1). Cochlear lateral wall length measurements were made per the reconstructed coronal FIESTA-C acquisition.
Internal acoustic canal 3D FIESTA-C protocol
Recording and Processing of the Demographic and Imaging Data
Demographic data of the study participants were recorded. CT and MR images of the patients were blinded and transferred to a Mac OS X (10.15.7; Apple) computer; raw imaging data were registered with the PACS software. Measurements were made with a 3D MPR application.
Two observers performed and recorded their CT and MR imaging measurements independently: Observer 1 had performed cochlear measurements on 387 cases before participating in the study; observer 2 had no prestudy experience of cochlear measurement and was given 1 hour of training with sample cases. First, they measured the cochlea on the CT images, followed by measurement on MR imaging at a gap of 2 weeks to avoid recognition bias.
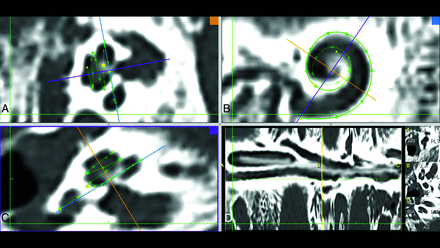
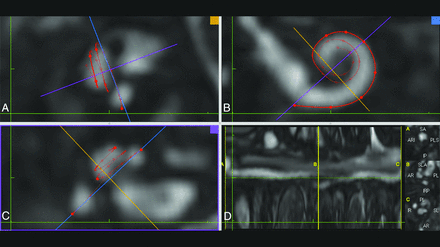
For CT measurements, the procedure described by Eser et al12 was used to measure the length of the cochlear duct starting from the cochlear view, following the full-turn cochlea, and covering the lateral cochlear wall. The observers selected each point as the outermost coordinate to avoid partial volume effects and beam-hardening artifacts (Fig 1). The MR imaging measurement method was modified to obtain the round window niche and follow the hyperintense cochlear fluid boundary (Fig 2). Unlike the previous study, the hook of the apical turn was not included for both CT and MR imaging measurements.12
Screenshot of the CT postprocessing software used in the study. A and C, Planes perpendicular to the modiolus are formed. B, The cochlear view is shown; in this plane, the basal turn can be fully traced and the bone structure of the round window is visualized as a thin line parallel to the purple line. The purple line also indicates the A-value measured from the niche to the opposite cochlear wall and the diameter of the cochlear basal turn. C, The round window niche is distinguished as a hypodense area below the measuring point. D, The measurement screen.
Screenshot of the MR imaging postprocessing software. A and C, Planes perpendicular to modiolus are formed. B, The cochlear view is shown; in this plane, the basal turn can be fully traced and the bone structure of the round window is viewed as a thin hypointense line parallel to the purple line. C, The round window niche is not distinguished, and bone and air are shown as hypointense on the FIESTA-C MR imaging sequence. D, The measurement screen.
Statistical Analysis
Gaussian distribution of the outcome data was evaluated using the Kolmogorov-Smirnov test, and Gaussian-distributed data were presented as mean (SD). A paired t test was used to determine the mean difference. Intraobserver and interobserver reliability for CT and MR imaging was evaluated using the intraclass correlation coefficient (ICC) (with 95% confidence intervals) as described by Koo and Li13 (2-way mixed-effects, absolute agreement, single measurement). If the ICC value was 0.49 and below, the reliability was considered weak. ICCs ranging from 0.50 to 0.74 represented moderate reliability, between 0.75 and 0.89 represented good reliability, and >0.90 meant excellent reliability. A P value < .05 was used to determine statistical significance. All data were evaluated using SPSS for Mac OS X (Version 22.0; IBM).
RESULTS
Demographic Data of the Participants
The study included 35 patients (all whites), of which 70% (n = 21) were men. The average age of all participants was 39.85 (SD, 16.60) years, while the mean age of male participants was 36.71 (SD, 15.02) years, and it was 44.57 (SD, 18.26) years for the female participants. Measurements on CT and MR images of 1 patient’s left ear could not be made due to calcifications secondary to chronic otitis media, so this ear was excluded. MR imaging of another 2 patients was excluded due to motion artifacts. Therefore, interobserver reliability analyses were performed on 69 ears for CT and 65 ears for MR imaging, while 65 ears were evaluated during intraobserver reliability analyses.
Cochlear Length Measurement and Intraobserver Reliability Results
Observer 1 measured the mean cochlear lateral wall length (LWL) as 41.52 (SD, 2.25) mm on CT and 41.44 (SD, 2.18) mm on MR imaging, with a mean difference for the full-turn cochlear length between these 2 observations as 0.08 mm (95% CI, −0.11−0.27 mm). The average cochlear LWLs and ICC results (with 95% confidence intervals) for observer 1 are presented in Table 2.
Intraobserver accuracy and reliability of observer 1’s cochlear LWL measurements between CT and MR imaging
Observer 2 measured the mean cochlear LWL as 41.74 (SD, 2.69) mm on CT and 42.34 (SD, 2.53) mm on MR imaging, with a mean difference of −0.59 mm (95% CI, −1.00 to −0.20 mm) between these observations for the full-turn cochlear length. The average measurement and ICC results (with 95% confidence intervals) for observer 2 are given in Table 3.
Intraobserver accuracy and reliability of observer 2’s cochlear LWL measurements between CT and MR imaging
Interobserver Reliability Results
An ICC value of 0.90 (95% CI, 0.84−0.94) for CT and 0.69 (95% CI, 0.46−0.82) for MR imaging was obtained for the interobserver reliability for the full-turn cochlear LWL. The reliability between the 2 observers as assessed by ICCs is given in Table 4.
Interobserver reliability of 2 observers
DISCUSSION
This study tested the accuracy and reliability of measuring cochlear length using a 3D reconstruction on CT and MR imaging. High intraobserver and interobserver reliability was observed for CT-based measurements, whereas good-to-excellent intraobserver reliability and moderate-to-good interobserver reliability was observed for MR imaging to measure cochlear LWL.
Accuracy and Reliability of 3D Cochlear Length Measurements
Intraobserver accuracy of an experienced observer’s CT measurements (observer 1) with MR imaging measurements was the following: There was moderate reliability for the basal turn length, good reliability for the 2-turn length, and excellent reliability for the full-turn cochlear length. There have been limited studies evaluating MR imaging for similar objectives.14,15 One of these studies measured the intraobserver and interobserver reliability of the A-value, while the other study used the spiral ganglion as the measurement target, and only 1 observer performed the measurements. Multiple studies have been performed with CT,3 and most measured the A-value and rarely dealt with the reproducibility of the method. Schurzig et al16 revealed the superiority of 3D measurements over spiral formulas. Likewise, Eser et al12 evaluated 3D CT measurements and reported good-to-excellent intraobserver reliability in their study (ICC = 0.87).
Our results show that the cochlear lateral wall length measurements are accurate when done by an experienced observer. The second observer had no experience in temporal bone imaging, except for 3 years of radiology experience and was given only 1 hour of training before measurements. A short training period was chosen as per the study hypothesis, which was that even with a short training period, there would be high accuracy and reliability. This hypothesis was proved for CT-based measurements: Good-to-excellent reliability was seen between the 2 observers. A probable reason is that the bony anatomic structures can be visualized easily with high-resolution CT (Fig 3). On the contrary, the section thickness is higher in MR imaging, making it more difficult to detect anatomic bone landmarks. However, while the interobserver reliability was moderate-to-good with MR imaging, our results demonstrate high (good-to-excellent) intraobserver reliability with MR imaging.
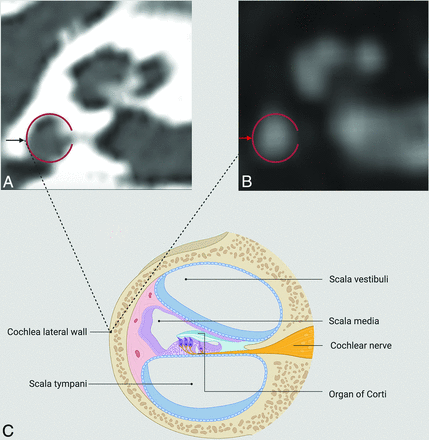
CT (A) and MR imaging (B) of the cochlea of the same patient. The red ring seen in these images shows the lateral wall of the cochlea. The black arrow seen on the CT (A) image and the red arrow seen on the MR imaging (B) image indicate the outermost point of the lateral cochlear wall. The lateral cochlear wall, the target point of measurement, and other anatomic structures are summarized in a schematic view (C). Created with BioRender.com.
Although Observer 1’s mean MR imaging measurements were similar to the CT measurements, the second observer’s MR imaging measurements were longer than the CT measurements, a probable reason being the basic difference between the 2 imaging techniques.12,14 The structure visualized on CT was the hyperdense bony cortex surrounding the cochlea, while on MR imaging, it was the hyperintense fluid in the cochlea. These may cause a partial volume artifact for both imaging modalities.12 Therefore, the cochlea can be observed slightly shorter than its actual size with CT and is slightly longer than its actual length with MR imaging. Second, the anatomic landmarks required for measurement are probably more clearly differentiated on CT.17 For instance, the round window niche is an anatomic landmark that has been used unanimously in previous studies as the starting point for CT measurement and can be easily detected in the cochlear view.18 In this study, both observers faced difficulty in finding the round window niche because it is made of a thin bone structure and was hardly differentiated on MR imaging due to the hyperintense fluid signal of the inner ear.
The final challenge that reduces interobserver reliability is the difficulty in deciding the cochlea apical end point. In 2013, Erixon and Rask-Andersen19 included the hook segment in the measurement and found an approximately 1-mm variation in the cochlear apical turn between the 2 observers. Moreover, the measurements were taken with a highly sensitive micrometer made from the plastic casts of cochleae obtained from cadavers. Despite an increase in the interobserver reliability with this choice, we decided to exclude the hook segment in the apical turn, contrary to previous studies.
Last, it is important to consider how measurements are affected by the observer’s experience. As hypothesized, the experienced observer’s intraobserver reliability reached an ICC value of 0.94, while the inexperienced observer had an ICC of 0.86, which was still highly acceptable. Furthermore, the interobserver reliability was better for CT-based measurements (ICC = 0.90), while it was moderate (ICC = 0.69) for MR imaging due to the aforementioned reasons. These results suggest that MR imaging measurements are affected more negatively by the observer’s experience than CT measurements.
Is the Difference in Interobserver Reliability Clinically Significant?
On evaluating paired t test results across the intraobserver and interobserver reliability, we observed that the highest difference achieved in the 2 observers’ MR imaging measurements was for the full-turn cochlear length (−0.90 [SD, 1.71] mm; 95% CI, −1.31 to −0.47 mm). This result shows that there was a negligible difference (on average, 1 mm) between the 2 observers. Considering that cochlear implant lengths are currently produced at 2-mm intervals, this difference is not clinically essential in cochlear implant selection.20 In a recent meta-analysis, Atalay et al3 reported a similar difference (0.61 [SD, 0.54] mm) in the organ of Corti length between people from the general population and patients with acquired hearing loss and stated that this difference was not significant with the same reasoning. However, Schurzig et al16 compared the accuracy of spiral formulas. For the same A-value, they found the organ of Corti length varying from 29.2 (SD, 2.30) mm to 43.4 (SD, 3.40) mm. Considering that 2 mm is important in this implant selection, it is far from an acceptable margin of error.3,9,16,20 Therefore, CT measurements should preferably be considered in the selection of cochlear implants, and MR imaging may be used as a second-line alternative.
How Do the Technical Differences between CT and MR Imaging Influence the 3D Cochlear Length Measurements?
Imaging technology is improving progressively. The most commonly used imaging method for pre-cochlear implant evaluation is multidetector CT, followed by conebeam CT.3 In a recent study, 0.25-mm isotropic-voxel resolution was achieved with ultra-high-resolution CT in clinical images.21 However, with MR imaging, the section thickness cannot go below 0.60-mm isotropic-voxel resolution, even with 3D sequences. Although this voxel size difference does not seem very large, 3D CT images have approximately 14 times higher spatial resolution than MRI21; this spatial resolution difference is probably responsible for the high interobserver reliability with CT. Although significant improvements have been made recently for bone imaging with MR imaging, such as zero TE, to reduce these technical differences, CT is more useful clinically.22,23 Nevertheless, if MR imaging sequences developed in the future can show the bone structure in detail, pre-cochlear implant imaging of patients can be performed with MR imaging only, significantly avoiding unnecessary radiation exposure. Extensive and comprehensive studies are needed to obtain a higher spatial resolution with MR imaging that allows detailed imaging of the bone structure.
Last, it is necessary to discuss the MR imaging sequences and magnets exclusively. Besides the 3D FIESTA-C and CISS sequences used frequently for imaging the internal auditory canal, cochlea, and labyrinth, 3D FSE sequences are also used.24⇓⇓⇓-28 The 3D T2 FSE sequences (Cube, GE Healthcare; sampling perfection with application-optimized contrasts by using different flip angle evolutions [SPACE], Siemens; driven equilibrium radiofrequency reset pulse [DRIVE]) can eliminate banding artifacts and have fewer flow and susceptibility artifacts.28 Also, the use of 3T magnets provides a high signal-to-noise ratio and better spatial resolution than 1.5T, with shorter acquisition times.24,25 However, more artifacts are encountered along with these advantages, especially with gradient recalled-echo sequences (banding and susceptibility artifacts). These artifacts may obscure fine anatomic detail of the cochlea and labyrinth.
Limitations
Our study has some limitations. Although the CT and MR imaging devices used in the study are currently the most used device technologies, ultra-high-resolution CT and 3T-magnet MR imaging devices are also available. Other researchers may repeat this study using currently available higher resolution isotropic 3D T2 sequences (Cube, SPACE, DRIVE) acquired at 3T. Studies with these new devices are likely to provide a greater interobserver reliability for MR imaging. If MR imaging scans were obtained in the cochlear view plane, the distortion-caused artifacts could be reduced, increasing the interobserver reliability. Another limitation is the difference in the level of experience between the 2 observers. However, to avoid this, we tested the effect of experience on measurements; high reliability in CT measurements showed us that the observer experience has a minimal effect on CT-based evaluations. Nevertheless, comparing MR imaging measurements of observers with comparable professional experience will help appreciate the true potential of MR imaging measurements.
CONCLUSIONS
This study showed that the 3D cochlear length measurements made with CT had excellent intraobserver and interobserver reliability, while MR imaging–based measurements also demonstrated good-to-excellent intraobserver reliability for full-turn cochlear measurement and moderate interobserver reliability. These results corroborate the importance of CT imaging in 3D cochlear measurements as a reference method, and MR imaging may be used as an alternative imaging technique that offers comparably reliable results.
Acknowledgment
Figure 3 was created with BioRender.com.
Footnotes
The data that support the findings of this study are available on request from the corresponding author.
Paper previously presented at: Annual Scientific Meeting of the European Society of Head and Neck Radiology, September 2–4, 2021; Virtual, Vienna, Austria.
References
- Received April 21, 2021.
- Accepted after revision July 25, 2021.
- © 2021 by American Journal of Neuroradiology