Abstract
BACKGROUND AND PURPOSE: Coloboma of the eye, Heart defects, Atresia of the choanae, Retardation of growth and/or development, Genital and/or urinary abnormalities, Ear abnormalities and deafness (CHARGE) syndrome is an autosomal dominant genetic disorder with evolving clinical diagnostic criteria. Recently, a number of additional anomalies have been described in this syndrome, which may aid in early diagnosis, particularly in incomplete phenotypes or atypical cases. The persistent trigeminal artery is an embryonic carotid-vertebral anastomosis, rarely seen in the healthy population, with a reported prevalence of 0.4%. Because we had observed the persistent trigeminal artery in patients with CHARGE syndrome, this study aimed to explore the prevalence of the persistent trigeminal artery in this syndrome.
MATERIALS AND METHODS: A retrospective study was performed at our tertiary center. MR imaging studies, clinical records, and genetic results were reviewed for patients diagnosed with CHARGE syndrome between 2006 and 2019. The prevalence of the persistent trigeminal artery in patients with CHARGE syndrome was recorded and compared with other established diagnostic criteria.
RESULTS: Twenty-five patients with CHARGE syndrome were included. The persistent trigeminal artery was demonstrated on MR imaging in 14/25 (56%) patients and was seen more frequently than 4 of 9 other established diagnostic criteria in our cohort. When individual major or minor diagnostic criteria were absent, the persistent trigeminal artery was still demonstrated on MR imaging in 52%–67% of these patients with CHARGE syndrome.
CONCLUSIONS: The prevalence of the persistent trigeminal artery in CHARGE syndrome of 56% is higher than that of some other established diagnostic criteria and much higher than that in the general population. The persistent trigeminal artery may be a useful addition to the expanding phenotype of CHARGE syndrome, supplementing other diagnostic criteria. Radiologists should be aware of this novel finding demonstrable on MR imaging.
ABBREVIATIONS:
- CHARGE
- Coloboma of the eye, Heart defects, Atresia of the choanae, Retardation of growth and/or development, Genital and/or urinary abnormalities, Ear abnormalities and deafness
- CHD7
- Chromodomain Helicase DNA-binding protein
- PcomA
- posterior communicating artery
- PHACE
- Posterior fossa/brain malformations, Hemangiomas, Arterial anomalies, Coarctation of the aorta, and Cardiac anomalies and Eye abnormalities
- PTA
- persistent trigeminal artery
Coloboma of the eye, Heart defects, Atresia of the choanae, Retardation of growth and/or development, Genital and/or urinary abnormalities, and Ear abnormalities and deafness (CHARGE) syndrome is a rare, usually sporadic, autosomal dominant disorder caused by heterozygous mutations in the chromodomian helicase DNA binding protein 7 (CHD7) gene in most cases. While the acronym CHARGE was based on the cardinal features identified when the syndrome was initially described, the clinical diagnostic criteria have since evolved. They now include the 3C triad (Coloboma-Choanal atresia-abnormal semicircular Canals), olfactory hypoplasia, and/or arhinencephaly and rhombencephalic dysfunctions, which are now considered the most important and constant clues to the diagnosis.1⇓⇓⇓-5
The persistent trigeminal artery (PTA) is an embryonic carotid-vertebral anastomosis that is normally transiently seen in early fetal life. Because it is rarely reported in the healthy population, with a prevalence of 0.4%,6 and is easily recognized on MR imaging, it would constitute a potentially useful syndromic association. Because we had observed the PTA on MR imaging studies in patients with CHARGE syndrome, our hypothesis was that the PTA would have a high prevalence in CHARGE syndrome and would represent a useful diagnostic criterion. Hence, our primary objective was to determine the prevalence of the PTA on MR imaging in patients with CHARGE syndrome, and our secondary objective was to compare the prevalence of the PTA with that of other diagnostic criteria.
MATERIALS AND METHODS
Patients
A retrospective study on CHARGE syndrome was performed at Guy's and St. Thomas' NHS Foundation Trust with local institutional approval and the need for informed consent was waived. The radiology information data base (CRIS, Healthcare Software Solutions, UK) was searched for MR imaging studies performed between 2006 and 2019 containing the terms “CHARGE-syndrome” or “CHARGE syndrome” in the report or clinical details. The clinical records and imaging studies were reviewed, and a diagnosis of CHARGE syndrome was confirmed on the basis of either genetics or a defined clinical diagnostic criterion subcategorizing into typical, partial/incomplete, or atypical CHARGE.5 The demographic data, clinical criteria, and results of genetic testing were recorded.
Imaging Analysis
Imaging was performed on both 1.5 (n = 21) and 3T (n = 6) MR imaging systems from the same manufacturer (Philips Healthcare), with 2 patients having undergone scanning on both systems. Most 1.5T MR imaging studies were performed on a single scanner, an Achieva 1.5T (Philips Healthcare), while the other studies were performed on a 3T Achieva scanner (Philips Healthcare). Axial T2WI was available for all patients (4- to 5-mm section thickness on 1.5T and 2-mm thickness on 3T). 20/25 patients also had additional thin-section (1- to 1.4-mm section thickness) heavily T2WI through the posterior fossa. Time-of-flight MR angiography was acquired in 6/25, including in 2 patients who did not have thin-section T2WI.
Imaging was reviewed by 2 experienced neuroradiologists (A.S. with 13 years' consultant/senior experience and S.E.J.C. with 18 years' consultant/senior experience), and agreement was reached by consensus. The presence of a PTA was recorded if a vessel was seen connecting the intracavernous ICA to the basilar artery. When present, the PTA was subclassified as either medial or lateral, depending on its origin from the medial or lateral surface of the ICA and/or a course relative to the abducens nerve or lateral border of the dorsum sellae. Hypoplasia of the basilar artery below the level of the PTA and the presence, absence or hypoplasia of the posterior communicating artery (PcomA) (when an MRA sequence was available) were also recorded.
Statistics
Statistical calculations were performed using SPSS Statistics software (IBM). The prevalence of the PTA, along with the other major and minor diagnostic criteria, in patients with CHARGE syndrome was calculated. The prevalence of each diagnostic criterion, along with the prevalence of the PTA was compared; 95% confidence intervals were derived using a 1-sample binomial (Clopper-Pearson) test. The Fisher exact test was used to compare PTA prevalence between defined groups. A P value < .05 was deemed significant.
RESULTS
Patient Characteristics and Diagnostic Criteria for CHARGE
Twenty-five patients (male = 16; female = 9) with a diagnosis of CHARGE syndrome were included in the study. Ages ranged from 2 days to 22 years (median = 1 month), with 12 neonates (0–1 months), 5 infants (1–12 months), and 8 older children/adults (1–22 years). Most neonatal MR imaging studies were performed for dysmorphology or cardiovascular problems, whereas MR imaging studies in infants and older children were performed for various other indications, including cranial nerve dysfunction and hearing loss. On the basis of clinical criteria,5 22/25 had a clinical diagnosis of typical CHARGE syndrome, 2/25 had atypical CHARGE syndrome (but confirmed genetically), and 1/25 had limited clinical information (also confirmed genetically). Genetic testing for mutations in the CHD7 gene was performed and confirmed in 17/25 cases; the remaining 8 cases had clinical confirmation of the diagnosis but had either not undergone genetic testing or the results were unavailable. With the Fisher exact test, there was no significant difference between the presence or absence of the PTA between the genetically confirmed and clinically confirmed cases (P = .5). Similarly, there was no significant difference in the prevalence of the PTA between patients with typical and atypical CHARGE syndrome (P = .7).
Imaging Findings of the PTA
The PTA was seen in 56% (n = 14) of patients with CHARGE syndrome (Figs 1 and 2). When seen, the PTA was medial in 79% (11/14) and lateral in 21% (3/14) of patients. The basilar artery below the PTA was hypoplastic in 36% (5/14) of cases, and the PcomA was absent or hypoplastic in all 6 patients who had undergone MRA. The PTA was seen in 8/14 males (57%) and 6/14 females (43%) and was unilateral in all patients. When present, it was also seen on standard 5mm thick-section T2WI of the head in 8/14 (57%). In 3 neonates who had undergone subsequent follow-up imaging in later childhood, the PTA was demonstrable on each occasion. The PTA was also seen in 4/8 patients who did not have genetic testing. Other imaging findings of CHARGE syndrome (Figs 1 and 2) were also recorded.
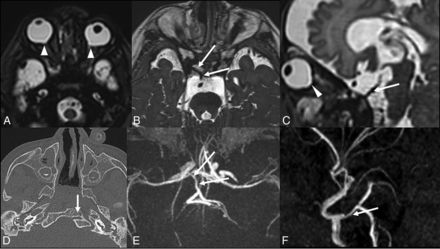
PTA in CHARGE syndrome, medial variant. Axial T2WI (A and B), sagittal T2WI (C), axial CT (D), and MRA transverse (E) and lateral (F) views in a neonate demonstrate a PTA (long arrows) connecting the intracavernous ICA to the basilar artery. Note the bilateral ocular colobomas (arrowheads), absent semicircular canals, and clival cleft (arrow in D).
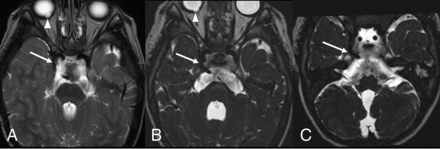
PTA in CHARGE syndrome, lateral variant. Standard 5-mm brain axial T2WI (A) and thin-section submillimetric T2WI (B and C) show a lateral-type PTA (long arrows), bilateral coloboma (arrowheads), and aplasia of the semicircular canals. The PTA is less clearly evident on the standard brain T2WI (A) compared with the thin-section images (B and C). Note the hypoplastic basilar artery below the PTA.
Comparison between the Presence of PTA and Other Diagnostic Criteria
The Table shows the comparison between the prevalence of the PTA and other diagnostic criteria in patients with CHARGE syndrome. In particular, the prevalence of the PTA (56%, 14/25) was higher than some of the established major and minor clinical criteria such as choanal atresia (40%, 10/25), gonadal anomalies (40%, 10/25), orofacial/laryngeal clefting (32%, 8/25), and tracheoesophageal fistula (8%, 2/25). Regarding an additional value when a major criterion was absent, the PTA was seen in 55% (5/9) of patients with CHARGE syndrome without documented colobomas and 53% (8/15) of patients without documented choanal atresia. Comparison with minor criteria also showed that the PTA was seen in 57% (4/7) of patients without cardiovascular anomalies, in 67% (10/15) of patients without gonadal anomalies, in 65% (11/17) of patients without orofacial/laryngeal clefting, and in 52% (13/23) of patients without a tracheoesophageal fistula, further supporting the additive complementary value of this finding.
DISCUSSION
The PTA was demonstrated on MR imaging in 56% (14/25) of cases in our cohort of patients with CHARGE syndrome. The prevalence of the PTA in patients with CHARGE syndrome was higher than 4 of 9 other established diagnostic criteria. The PTA was still present in most patients in whom specific major criteria (colobomas and choanal atresia) or minor criteria (cardiovascular anomalies, gonadal anomalies, orofacial/laryngeal clefting, tracheoesophageal fistula) were absent.
CHARGE Syndrome
CHARGE syndrome (or Hall-Hittner syndrome) was first described as an association of congenital anomalies in 1979.1,2 The acronym CHARGE is based on the cardinal features identified when the syndrome was delineated: Coloboma, Heart malformation, choanal Atresia, Retardation of growth and/or development, Genital and/or urinary anomalies, and Ear anomalies.3 Diagnosis is based on clinical criteria, which have been updated across time, with emphasis on the 3C triad (Coloboma, Choanal atresia, and abnormal semicircular Canals).4,5 It was subsequently labeled as the CHARGE syndrome because the association of multiple anomalies was pathogenetically related following the identification of the CHD7 gene.7 Since that time, several new clinical findings have been added to the clinical spectrum of CHARGE syndrome, such as dysmorphic features, rhombencephalic dysfunction, cranial nerve dysfunction, hypoplasia of the semicircular canals, olfactory hypoplasia and/or arhinencephaly, feeding difficulties, esophageal anomalies, facial clefts, and hypothalamic-hypophyseal dysfunction.8⇓⇓⇓⇓-13 The clinical spectrum of CHD7 mutations and CHARGE continues to expand, and recent literature has focused on the presence of venous and skull base anomalies.14,15
Genetically, CHARGE syndrome is a monogenic autosomal dominant disorder with an incidence of approximately 1:8500 to 1:12,500 in North America.5,12,16 It results from a dysblastogenetic and dysneurulative process and could be related by a common pathogenetic mechanism resulting in disturbed neural crest development.12 Mutations in the gene CHD7 (in 8q12) were identified as causative for CHARGE syndrome in most (approximately two-thirds) patients with a clinical diagnosis of CHARGE syndrome.7 CHD7 belongs to a large family of evolutionarily conserved proteins thought to play a role in chromatin organization and is a regulatory element that potentially affects a large number of developmental pathways, explaining the pleiotropic nature of its phenotypic spectrum.12
While congenital cardiovascular anomalies are commonly seen in CHARGE syndrome, anomalies of the head and neck vessels have been rarely described, with occasional case reports of isolation of the carotid artery, isolation of the left subclavian artery from the pulmonary artery, and complex cervical arteriovenous fistulas.17⇓-19
PTA
The PTA is one of the primitive carotid-vertebrobasilar anastomoses; it is the largest of these anastomotic vessels seen in fetal life and is seen in approximately 0.1%–1% of angiographic studies,20,21 with a recent meta-analysis demonstrating a pooled prevalence of 0.4%.6 In utero, the trigeminal artery supplies the basilar artery before development of the PcomA and vertebral arteries, with subsequent involution. The PTA arises from the junction between the petrous and cavernous ICA and runs posteriorly along the trigeminal nerve or crosses over or through the dorsum sellae. Vertebral, posterior communicating, and caudal basilar arteries are often hypoplastic. In 1959, Saltzman22 reported 8 cases and proposed an angiographic classification for the PTA into 2 main types. In Saltzman type 1, or the fetal variant of the PTA, the basilar artery proximal to the insertion of the PTA may be hypoplastic and the PcomA may be absent, making the PTA a very important vessel supplying the entire basilar artery system distal to the anastomosis and providing flow to a large part of the posterior circulation, whereas in Saltzman type 2, or the adult variant, the PTA is a relatively less important vessel, with the posterior cerebral arteries receiving their blood supply predominantly through patent PcomAs and the basilar artery filling by the vertebral arteries.21,23 While we were not able to classify all PTAs according to the Saltzman system, in our series, the basilar artery was hypoplastic below the PTA in 36% (5/14) of patients and the PcomAs were hypoplastic in 6/6 patients who had undergone an MRA, suggesting that these were probably type 1, thereby making the PTA a potentially significant source of supply to the posterior circulation.
The exact etiology of the persistence of this vessel into postnatal life is unclear. The PTA is the most prominent of the carotid-basilar anastomoses, existing in early fetal life (4- to 5-mm embryonic stage) and providing an important source of flow to the developing rhombencephalon, arising from the developing ICA to connect with the paired longitudinal neural arteries, which would eventually form the basilar artery. Growth of the posterior fossa structures, interposition of the developing basisphenoid cartilages, and evolution of the carotid and vertebrobasilar systems result in obliteration of the PTA at around the 7- to 14-mm embryonic stage, with the PcomA superseding the PTA from above and the vertebral arteries developing from below.21 The PTA has been reported in association with vascular malformations, aneurysms, trigeminal neuralgia/compression, and PHACE syndrome, with additional implications in surgical and endovascular treatment-planning, making it a relevant finding to note, with significance potentially emerging later in life.20,21,24 One of our patients had a mild ICA hypoplasia contralateral to the PTA, but we did not find any vascular malformations or aneurysms in our study.
Association of the PTA and CHARGE Syndrome
To the best of our knowledge, this is the first report describing a compelling association of the PTA with CHARGE syndrome. This finding was readily identified on imaging studies and particularly when thin-section T2WI was available. While it is important for the neuroradiologists to recognize this association, it may also have an additional supplementary value when other criteria are lacking, particularly in cases of atypical or partial/incomplete CHARGE syndrome. The presence of the PTA on neuroimaging studies, particularly in the context of a child with complex cardiovascular anomalies or rhombencephalic dysfunction, should prompt a review for other hitherto unsuspected imaging features of CHARGE syndrome.
The prevalence of PTA of 56% (14/25) in cases of CHARGE syndrome is particularly high, given the relatively low prevalence of the PTA (0.4%).6 Similarly, we have not found any reference to the PTA or intracranial arterial anomalies in the context of CHARGE syndrome, including large reviews on this subject.25,26 This could potentially be due to the lack of thin-section imaging of the posterior fossa or lack of MR angiographic imaging. The PTA was clearly identified on the thin-section T2WI performed for assessment of the inner ears or when thin-section T2WI of the head was available, but it could also be demonstrated in 57% (8/14) on the standard T2WI of the head (Fig 2). There was no particular correlation with sex. We also found that a substantial proportion of patients with CHARGE syndrome, who did not have one of the major or minor diagnostic criteria, had the presence of the PTA, as shown in the Table, in more than half of the cases. This is a strong argument for including the PTA as an additional diagnostic criterion of CHARGE syndrome, to further support the other diagnostic criteria, increase diagnostic confidence, and direct appropriate investigations. Indeed, 2 patients who did not have a typical CHARGE diagnosis (but were genetically confirmed) had a PTA.
CHD7 is expressed ubiquitously in fetal and adult tissues, including eye, olfactory epithelium, inner ear, and the vascular system.7 The in situ hybridization analysis of the CHD7 gene during early human development has shown a good correlation between the specific CHD7 expression pattern and the developmental anomalies observed in CHARGE syndrome.12 Therefore, it is plausible that mutations in this gene in some could result in vascular maldevelopment and persistence of embryonic vessels. Embryologically, the PTA is a significant source of supply to the developing hindbrain in very early fetal life at around 4–6 weeks but has a very limited time span of approximately a week or so, with normal regression of the PTA paralleling the simultaneous development of the PcomA and vertebral arteries, which then take over the function of providing flow to the posterior circulation and the rapidly developing hindbrain.21,27,28 It is also well-established that hindbrain dysfunction is a characteristic feature of CHARGE syndrome; therefore, the finding of parallel anomalous vascular development is not surprising. Embryologically, CHARGE is thought to be a complex neurocristopathy, related to abnormalities of neural crest cells and abnormal differentiation of the cephalic mesoderm and ectoderm occurring between 3 and 9 weeks of gestation.5 This timing also corresponds to and overlaps the time of development and regression of the PTA, thereby potentially explaining its persistence in CHARGE.
PTA versus Other Criteria in CHARGE
Compared with the other accepted diagnostic criteria, the PTA fares well, and the prevalence of PTA in our series (56%, 14/25) is higher than some major and minor criteria (Table). It is well-documented that semicircular canal aplasia is seen in almost all cases of CHARGE syndrome, as was seen in 100% of our cases. However, regarding other major criteria, ocular colobomas were seen in only 64% (16/25), and choanal atresia, in 40% (10/25) of our cohort. Previous studies have indicated the prevalence of ocular colobomas to be 75%–90% and choanal atresia to be 35%–65%.12 The prevalence of minor criteria in our study was variable, and the PTA also compared favorably. This finding is a strong argument in support of including the PTA as an additional diagnostic criterion. It is also topographically distinct from the other established clinical criteria and adds the missing arterial angle to the pre-existing broad clinicoradiologic phenotype of CHARGE syndrome. In addition to our findings of the high prevalence of the PTA, recent studies have also demonstrated a high prevalence of clival pathology in CHARGE syndrome, with characteristic clival clefts found in nearly 90% of cases, and cerebellar malformations/heterotopia, in >70%, further arguing for expansion of the existing clinical criteria.15,29,30
Limitations
There are limitations to a retrospective study. In particular, there were variable MR imaging sequences and technical parameters, and MRA was available in only 6/25 patients. However, most of our patients (20/25) had undergone thin-section T2WI, primarily for assessment of the vestibulocochlear nerves and inner ears, and the PTA was well-demonstrated on this sequence. In 3 patients without MRA or thin-section T2WI, the axial head T2WI did not show an obvious PTA, and we acknowledge the possibility that a small PTA may have been present. When one compares the utility of the PTA with other diagnostic criteria, one must consider their prevalence in other syndromic and nonsyndromic populations. We did not look at the prevalence of the PTA in other syndromes or similar conditions; therefore, we are unable to assess the specificity of this finding and how frequently it is present in other related syndromes, though we did not find any reference to the PTA in chromosomal syndromes overlapping with CHARGE syndrome such as 22q11.2 deletion or cat eye syndromes. We also acknowledge that the available clinical data may have been limited in some of our patients. Additional prospective multi-institutional studies would be helpful in further validation of this interesting finding and bringing into focus the association of arterial anomalies with CHARGE syndrome, which may have significance in later life.
CONCLUSIONS
This is the first report of an association between the PTA and CHARGE syndrome. With the identification of a high prevalence of the PTA in CHARGE syndrome (56%), which is higher than some other established diagnostic criteria and much higher than in the general population, our study adds another interesting and topographically distinct imaging finding to the existing broad phenotype of CHARGE syndrome.
Prevalence of PTA versus other CHARGE clinical criteria and of the PTA in cases in which the clinical criterion was absent
Footnotes
Disclosures: S.E.J. Connor—UNRELATED: Grants/Grants Pending: I have received a grant for MRI research on inner ears from the Royal College of Radiologists (Kodak Radiology Fund Scholarship) but not connected with this work.* *Money paid to the institution.
References
- Received March 21, 2021.
- Accepted after revision May 18, 2021.
- © 2021 by American Journal of Neuroradiology