Abstract
BACKGROUND AND PURPOSE: Delayed leukoencephalopathy is a rare complication that occurs after endovascular coiling of cerebral aneurysms. We aimed to describe a clinical picture of delayed leukoencephalopathy and explore potential associations with procedural characteristics.
MATERIALS AND METHODS: We considered endovascular coiling procedures for cerebral aneurysms performed between January 2006 and December 2017 in our institution with follow-up MRIs. We used logistic regression models to estimate the ORs of delayed leukoencephalopathy for each procedural characteristic.
RESULTS: We reviewed 1754 endovascular coiling procedures of 1594 aneurysms. Sixteen of 1722 (0.9%) procedures demonstrated delayed leukoencephalopathy on follow-up FLAIR MR imaging examinations after a median period of 71.5 days (interquartile range, 30–101 days) in the form of high-signal changes in the white matter at locations remote from the coil mass. Seven patients had headaches or hemiparesis, and 9 patients were asymptomatic. All imaging-associated changes improved subsequently. We found indications suggesting an association between delayed leukoencephalopathy and the number of microcatheters used per procedure (P = .009), along with indications suggesting that these procedures required larger median volumes of contrast medium (225 versus 175 mL, OR = 5.5, P = .008) as well as a longer median fluoroscopy duration (123.6 versus 99.3 minutes, OR = 3.0, P = .06). Our data did not suggest that delayed leukoencephalopathy was associated with the number of coils (P = .57), microguidewires (P = .35), and guiding systems (P = .57).
CONCLUSIONS: Delayed leukoencephalopathy after coiling of cerebral aneurysms may have multiple etiologies such as foreign body emboli, contrast-induced encephalopathy, or hypersensitivity reaction to foreign bodies.
ABBREVIATION:
- DL
- delayed leukoencephalopathy
Endovascular coiling is an effective procedure for preventing rupture of cerebral aneurysms or treating ruptured aneurysms.1,2 However, a small percentage of patients experience complications,3 which include thromboembolic events (incidence rate of 4.7%–12.5%) and aneurysm rupture (incidence rate of 2.0%–8.8%),3 along with posterior reversible encephalopathy syndrome4,5 and perianeurysmal edema, which may be indicative of symptomatic inflammatory reactions.6,-,8 Recently, delayed leukoencephalopathy (DL) has emerged as a new type of complication under several names, including delayed leukoencephalopathy,9 delayed enhancing lesions,10 and delayed multiple white matter lesions.11 Its various suggested etiologies include granulation reaction caused by foreign body emboli from the hydrophilic coating of procedural devices,10,12,-,15 contrast-induced encephalopathy,16,17 and nickel11 or bioactive polyglycolic/polylactic acid coil sensitivity.9,18 Despite an increasing number of reported cases, the overall clinical picture (natural history, incidence, onset time, symptoms, treatment, mortality, and morbidity) of DL remains unclear.
We aimed to define the clinical features of DL and investigate its possible associations with procedural characteristics in a retrospective study of cerebral aneurysms treated by endovascular coiling.
MATERIALS AND METHODS
Study Design and Participants
The internal review board of Jikei University Hospital approved this study (approval number 29–228 [8844]). All data related to the management of cerebral aneurysms were extracted from a comprehensive observational data base after approval of an internal review board. Each patient provided comprehensive written consent for the procedures and examinations. Specific informed consent for this study was waived because the data were obtained from routine examinations and treatments and analyzed retrospectively. At the request of the internal review board, we posted a notice of the study and gave patients an opportunity to refuse participation.
Patients with aneurysms (20 years of age or older) who underwent endovascular coiling and a follow-up MR imaging examination after 3, 6, and 12 months at our institution, between January 2006 and December 2017, were considered. For each procedure, patient age and sex, aneurysm location, largest diameter of the aneurysm, fluoroscopy time, and the type and volume of iodinated contrast medium, along with the type and number of devices used in the procedure, were extracted. Devices were defined as guiding systems (ie, guiding sheaths, guiding catheters, and median catheters), microcatheters (including balloon catheters), microguidewires, and coils. We did not consider whether the devices were used simultaneously. Furthermore, we did not consider stents and flow diverters in our analysis. Patients with DL were reviewed for their clinical history, which included MR imaging findings, neurologic findings, treatment of DL, final clinical outcome (minor morbidity, major morbidity, mortality), other procedural complications, allergy tests, time of onset of DL, and time of disappearance of DL.
Procedure
Endovascular coiling for all aneurysms was performed using digital subtraction angiography with a standard iodine contrast agent following our clinical routine.19,20 Patients received general anesthesia and systemic anticoagulation with heparin. The type and number of devices were customized during each procedure. Double-catheter, balloon-assisted, or stent-assisted techniques were used to prevent coil herniation into the parent artery in cases of wide-neck aneurysms.
MR Imaging
Follow-up MR imaging examinations were performed using a 1.5T or 3T MR imaging system, routinely including DWI, FLAIR, T1WI, T2WI, and TOF-MRA to identify possible recanalization of the treated aneurysm or postprocedural complications. Contrast-enhanced MR imaging was added at 12 months after the procedure at the discretion of the attending physician.
We considered the following MR imaging outcomes associated with DL:
Broad high-signal changes on FLAIR images in the white matter at regions remotely located from the coil mass and observed at ≥2 weeks after the procedure, while the DWI findings remained normal. (These findings were not present immediately after the procedure.)
Decreases in size or the disappearance of high-signal changes on FLAIR images within a few months.
Statistical Analyses
Descriptive statistics were used to summarize the results. Distribution of each variable was assessed using normal quantile plots. DL proportions were calculated for each patient and procedural characteristic. We assessed the association of DL with the number of guiding systems, microguidewires, and microcatheters by classifying the procedures into 2 groups, using 1 device or >1 device. The number of bioactive polyglycolic/polylactic acid coils per procedure was classified as no such coil used and ≥1 such coil used. The association of DL with the variables of age, largest aneurysm diameter, contrast volume, fluoroscopy time, and number of coils was assessed by classifying them into 2 groups of smaller than or equal to or larger than the median of the variable. ORs for the occurrence of DL were estimated by logistic regression. The evidence of an association was assessed using the Fisher exact test. Commercial software was used for the analyses (STATA, Release 15; StataCorp, College Station, Texas).
RESULTS
Patient Demographics
Our institution performed 1754 endovascular coiling procedures for 1594 cerebral aneurysms (193 ruptured, 1401 unruptured) between January 2006 and December 2017. The flow diagram regarding the selection of the procedures is presented in Fig 1. Three patients (younger than 20 years of age), 14 procedures that did not have any data regarding procedure devices, and 15 procedures that did not include follow-up MR imaging examination were excluded. There were 1722 procedures in the final analysis.
Flow diagram of procedure inclusion.
The DL incidence was 0.9% (1706 procedures without DL and 16 with DL) during the study duration. Among the 16 aneurysms in the 16 procedures (16 patients) with DL, there were 1 ruptured and 15 unruptured aneurysms. Adjunctive to the endovascular coiling procedure, 1 aneurysm was treated using the stent-assisted technique; 1 with the balloon-assisted technique; and 14 with the double-catheter technique. DL patient demographics are presented in Table 1, and the associated details are listed in On-line Table 1.
Patients with delayed leukoencephalopathy—aneurysm location, size, detection to disappearance intervals, postprocedural symptoms, treatment, and allergy test
Clinical Course
Fourteen patients with DL underwent endovascular treatment without intraprocedural complications and were neurologically intact without demonstrating abnormal MR imaging outcomes on the first day postsurgery. Among the 2 other patients with DL, one with an ICA-ophthalmic artery aneurysm demonstrated a worsening of visual acuity and evidence of central retinal artery occlusion. The patient was discharged 5 days after the procedure with mRS 1 (quarter blindness). The other presented with intraoperative aneurysm rupture; however, the coiling procedure could be completed after the rupture. The patient presented with a headache on the next day, and MR imaging revealed a minor subarachnoid hemorrhage. The patient was discharged after 8 days postsurgery without neurologic deficits.
DL symptoms or abnormal MR imaging findings were identified after a median period of 71.5 days (interquartile range, 30–101 days). Among the 16 patients with DL, there were 7 symptomatic and 9 asymptomatic patients. Among the symptomatic patients, 7 developed neurologic symptoms, 1 demonstrated continuous headache after discharge, and 6 developed motor weakness (1 with aphasia, 1 with visual field loss). These patients recovered from their symptoms after being treated with either steroid therapy or free radical scavenger. The 9 asymptomatic patients recovered without medical intervention. There were no minor or major continuous morbidity or mortality. Among the 16 patients with DL, 7 underwent a skin patch test for metal hypersensitivity and 3 tested negative, while 4 tested positive (Table 1).
MR Imaging Findings
Follow-up of 4 of the 16 patients with DL included contrast-enhanced MR imaging. On the basis of abnormal MR imaging findings, all 16 patients were diagnosed with DL.
A follow-up MR imaging examination for each patient was continued until there was an improvement in the abnormal findings. High-signal changes on FLAIR images for 13 patients disappeared after a median period of 229 days (interquartile range, 200–360 days) after undergoing endovascular coiling. Minor levels of high-signal changes persisted in the remaining 3 patients; however, there was a noticeable decrease in the size of the high-signal region.
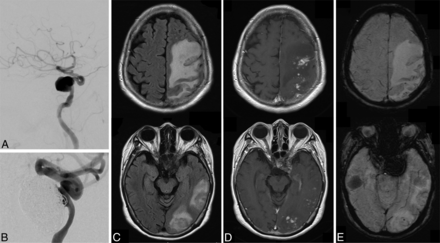
An illustrative case of a patient with DL whose follow-up included contrast-enhanced MR imaging is presented in Fig 2. DWI outcomes revealed no abnormal changes, but there were extensive high-signal changes on FLAIR images in a region that was primarily perfused by the vessel that underwent the endovascular procedure. Additionally, SWI outcomes demonstrated dot-shaped low signals in the regions with high-signal changes.
An illustrative case with left ICA aneurysm post-endovascular coiling (patient 11). The aneurysm size is >17 mm in maximum diameter (A), and endovascular coiling was successfully performed using the double-microcatheter technique (B). After 55 days, right weakness developed and broad high-signal change in the left parieto-occipital region is detected on a FLAIR image (C). In the same region, there is a scattered contrast effect (D) and low spotty regions in the SWI (E). The patient was hospitalized and treated with steroid pulse therapy, and the symptoms and image abnormality subsequently improved.
Procedural Characteristics
Procedural characteristics are summarized in Table 2 according to DL occurrence; and the associated DL odds ratios for each procedural characteristic, in Table 3. Details regarding the devices used during the procedures of patients with DL are listed in On-line Table 1, as well as the total number of devices by manufacturer used in our hospital (On-line Table 2, guiding systems; On-line Table 3, microcatheters; On-line Table 4, microguidewires; and On-line Table 5, coils) for the procedures with and without DL. We used a Shuttle sheath (Cook, Bloomington, Indiana) in 2 of the 16 patients with DL, bioactive polyglycolic/polylactic acid coils in 6, and 2 microcatheters simultaneously via 1 guiding system in all patients with DL. Use of >180 mL of contrast medium was associated with higher DL odds (OR = 5.5; 95% CI, 1.6–19.5; P = .008) and use of >1 microcatheter (P = .009, Fisher exact test). Our results also suggested that median fluoroscopy time longer than 99.5 minutes was associated with higher DL odds (OR = 3.0; 95% CI, 1.0–9.5; P = .06). We could not confirm an association of the use of bioactive polyglycolic/polylactic acid coils and higher odds of DL (OR = 0.4; 95% CI, 0.1–1.1; P = .08).
Patient and procedure characteristics (per procedure) with and without DLa
Odds ratio of complications for each patient and procedure characteristic (per procedure) with and without DLa
DISCUSSION
One of the strengths of our study is the large number of endovascular coiling procedures and patients with DL. We found that 0.9% of endovascular coiling procedures resulted in patients with DL and that their clinical course was typically benign.
The exact mechanism of DL remains uncertain; however, different hypotheses have been proposed. Some case reports indicated that DL may be a granulation reaction etiologically associated with foreign body emboli from the hydrophilic coating of the devices.10,12,-,14 Polymer coating embolism from intravascular devices has also been increasingly reported with regard to cardiovascular21 and endovascular therapy.22,23
In a previous study analyzing a series of 3 cases with delayed enhancing lesions—each treated with the double microcatheter technique—Oh et al10 performed a benchtop evaluation of the inner lumen of microcatheters. They found material wearing off the catheter shafts after multiple in-and-out coil maneuvers and proposed that DL may be caused by the fragmentation of the inner wall of microcatheters and consecutive emboli. Shapiro et al12 reported 5 patients who underwent coil-supported Pipeline Embolization Device (Covidien, Irvine, California) embolization or stent-supported endovascular coiling and presented with foreign body emboli after the procedure. Multiple catheters were used to treat most of the patients evaluated in our study because all endovascular coiling procedures involved stent or flow-diverter deliveries and all patients with DL were treated with multiple microcatheters. Therefore, our results also suggest that the hydrophilic coating of catheters may peel off and act as an embolic source due to the friction between the tight-fitting multiple catheters. The peeling off of the device coating may be associated with the complexity of these procedures, which may be reflected by a tendency of longer fluoroscopy duration in the procedures for patients with DL. In vitro experiments with multiple catheter types and frequent replacement and repositioning are required for further investigation. We routinely used guiding systems with a fairly large inner lumen (up to 8F), but choosing even larger catheters may further lower the friction between devices.
The neurotoxicity of iodinated contrast medium, along with the disruption of the blood-brain barrier and leakage into the brain tissue, has previously been reported to be associated with contrast encephalopathy.16,17,24 Contrast-induced encephalopathy typically follows coronary angiography and intracranial endovascular treatment and is characterized on plain CT as leakage of contrast medium.25,-,27 Transient cortical blindness is a commonly reported complication due to contrast-induced encephalopathy and is considered to be associated with high doses of contrast agents.26,28 Our study estimated that the odds for experiencing DL were higher for larger volumes of contrast agent (>180 mL). The patients in our study did not undergo CT examinations; however, 1 patient with DL had high-signal changes on FLAIR images in the hemisphere opposite the treated vessel, and it is possible that the contrast medium may have been translocated to this location. These results support a hypothesis that using a higher dose of contrast medium than the patient can metabolize may be associated with DL.17
Nickel allergy or hypersensitivity to polyglycolic/polylactic acid coils is reported to potentially cause DL.9,29 In our study, only 7 patients with DL received patch tests for metal hypersensitivity, and none of these 7 patients had metal allergies associated with nickel. We used polyglycolic/polylactic acid coils for only 6 of the 16 patients with DL. However, a hypersensitivity reaction to any foreign body used during the invasive procedures continues to be a possible etiology of DL.
It is our understanding that DL may be caused by various factors: foreign body emboli–associated granulation reaction, contrast-induced encephalopathy, or a hypersensitivity reaction to foreign bodies leading to identical image findings. We also cannot exclude interactions and associations between factors such as contrast-induced permeability and the retention of shaved-off catheter coating and the subsequent development of foreign body emboli.
Our study has several limitations. It was a single-center retrospective investigation using data collected from clinical routine procedures. The procedures included stents or flow diverters but not Woven EndoBridge devices (WEB; Sequent Medical, Aliso Viejo, California). There were only a few cases of DL despite the cases being relatively numerous compared with previous studies. None of our patients with DL had posterior circulation aneurysms, which may be related to sample size bias because DL has also been previously reported in such patients.11 The number of cases of DL was too few to apply a multivariate logistic regression, and we could not investigate confounding of the procedural characteristics. Pathology tests were not performed. Only a few patients with DL underwent contrast-enhanced MR imaging, and none underwent CT. Our analysis could not distinguish between the simultaneous and consecutive use of microcatheters. We could not investigate associations of specific devices with DL because different types of guiding systems, microcatheters, microguidewires, and coils and their combinations were customized during each procedure, which resulted in unique combinations in almost every procedure.
CONCLUSIONS
DL is a rare complication following endovascular coiling of cerebral aneurysms. We believe that it may be associated with several etiologies, including foreign body emboli related to complicated procedures or contrast-induced encephalopathy or hypersensitivity reaction to foreign bodies. Although the clinical course of DL is mostly benign, clinicians need to be aware of the potential causes of DL.
Acknowledgments
The authors wish to thank Ms Rie Sakima for clinical data preparation and the study team and all the study participants.
Footnotes
Disclosures: Katherine Otani—UNRELATED: Full-time employee of Siemens Healthcare K.K. Katherine Otani supported this study as a biostatistician. Yuichi Murayama—UNRELATED: Consultancy: Stryker, Kaneka Medix; Grants/Grants Pending: Stryker, Siemens K.K., Japan, Comments: research grant*; Payment for Lectures Including Service on Speakers Bureaus: Stryker, Cerenovus, Kaneka Medix; Royalties: Stryker.* *Money paid to the institution.
Paper presented, in part, at: Annual Meeting of the Japan Neurosurgical Society, October 10–12, 2018, Sendai, Japan; and Annual Meeting of the Japanese Society for Neuroendovascular Therapy, November 22–14, 2018; Migyagi, Japan.
References
- Received September 9, 2019.
- Accepted after revision November 27, 2019.
- © 2020 by American Journal of Neuroradiology