Abstract
SUMMARY: “Asleep” deep brain stimulation using general anesthesia and intraoperative MR imaging guidance is considered “off-label” use by current FDA guidelines but is widely used in neurosurgical practice, and excellent safety has been demonstrated using first-generation, omnidirectional electrodes. Safety data for second-generation, directional electrodes in the interventional MR imaging environment have not yet been published. Herein, we report 34 cases of asleep deep brain stimulation using second-generation, directional electrodes in an interventional MR imaging suite at a single institution. Procedural complications and imaging data are described. All patients underwent postoperative MR imaging with fully implanted (“internalized”) electrodes after scalp closure; 4 patients also underwent MR imaging with “externalized” electrodes before scalp closure. No MR imaging–related complications were observed, and procedural complication rates were comparable to prior series. This suggests that the use of second-generation, directional electrodes in the interventional MR imaging environment appears to be safe when following manufacturer-published imaging guidelines.
ABBREVIATIONS:
- DBS
- deep brain stimulation
- ET
- essential tremor
- GPi
- globus pallidus internus
- iMRI
- interventional MRI
- PD
- Parkinson disease
- SAR
- specific absorption rate
- STN
- subthalamic nucleus
- WMn
- white matter-nulled
- Vim
- ventralis intermedius
Deep brain stimulation (DBS) is a well-established treatment for patients with movement disorders such as Parkinson disease (PD), essential tremor (ET), and dystonia. Its use is expected to increase after recent FDA approval for the treatment of medically refractory epilepsy, and DBS targets are under investigation for numerous other neurologic diseases.1 DBS placement is typically performed in a standard operating room, with electrode placement guided by either physiologic mapping under local anesthesia (“awake DBS”) or intraoperative CT imaging under general anesthesia (“asleep DBS”). For postoperative imaging, existing DBS devices have “MR imaging-conditional” FDA labeling, meaning that MR imaging scans can be safely performed on patients with implanted systems under specific device and MR imaging conditions.
A newer method of asleep DBS placement is the use of intraprocedural MR imaging guidance, under sterile conditions and general anesthesia, within a diagnostic MR imaging suite. “Interventional MR imaging” (iMRI) DBS, like standard CT-based asleep DBS, has the advantages of greater patient comfort than awake surgery and real-time confirmation of electrode location. Large series of iMRI-DBS using first-generation, omnidirectional DBS electrodes (eg, Medtronic Model 3387/3389) have been published, demonstrating an excellent safety profile.2⇓⇓-5 However, despite the existing safety data and widespread acceptance into clinical practice, iMRI-DBS is still considered “off-label” use with respect to FDA approval of DBS electrodes. In addition, few published safety data exist regarding iMRI placement of second-generation, segmented (or “directional”) DBS electrodes, and the specific scenario of iMRI-DBS imaging performed with the electrodes in place but not yet secured under closed scalp incisions (“externalized” electrodes) is not addressed under MR imaging-conditional labeling, though these images are routinely obtained at some centers.
Given the discrepancy between practice and formal labeling, centers performing iMRI-DBS with segmented electrodes may therefore operate under restrictions for intraprocedural scanning, and institutional MR imaging safety committees have few data for guidance in these circumstances. There is consequently a need for postmarketing safety data on the use of iMRI for the placement of next-generation directional electrodes, as evidenced by the ongoing postmarket study by Abbott Medical Devices on the MR imaging safety of its Infinity DBS system (Abbott Neuromodulation).6
We present a single-institution study of 34 patients undergoing iMRI-DBS placement of Infinity segmented electrodes by using the ClearPoint system (MRI Interventions), including intraoperative imaging data and immediate perioperative safety outcomes.
MATERIALS AND METHODS
Data Collection
Following the Consensus Preferred Reporting Of CasE Series in Surgery (PROCESS) guidelines,7 a single-surgeon, prospectively maintained database was retrospectively analyzed to identify all patients undergoing asleep, iMRI-guided placement of Infinity DBS electrodes using the ClearPoint system from September 1, 2017, to January 31, 2020. The research setting is an academic neurosurgical practice in Tucson, Arizona.
Clinical data were abstracted from the database and medical record review was conducted by the first (N.G.) and senior (W.S.K.) authors. MR imaging parameters were abstracted from intraprocedural MR imaging protocols by the senior author and an experienced MR imaging physicist (M.S.). To identify complications, the official reports of intraoperative and postoperative imaging were reviewed, and each case was separately reviewed by an expert neuroradiologist (J.B.). For the purposes of this study, a complication was defined as any adverse event involving the intracranial electrodes within 30 days of surgery.
Patient Selection for Surgery
Patients with medically refractory movement disorders were selected for DBS surgery after discussion at a multidisciplinary movement disorders conference consisting of, at minimum, the treating neurologist and neurosurgeon. Patients with PD and dystonia underwent preoperative neuropsychological screening; patients with ET did so at the treating neurologist’s discretion. Our institutional shift from the Activa DBS system (Medtronic) to the Infinity system occurred in September 2017.
Surgical Procedure
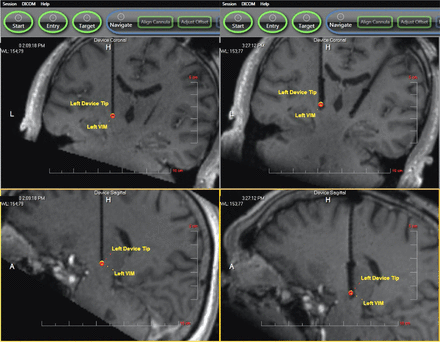
The procedure for iMRI-DBS placement has been described in detail elsewhere.8 In brief, after the induction of general anesthesia, the patient’s head is fixed to the MRI gantry with a custom 4-pin head holder (MRI Interventions). After sterile preparation and draping, the skull entry site is marked through the skin, the scalp is opened, and a skull-mounted miniframe (SmartFrame, MRI Interventions) is rigidly affixed using bone screws. MR imaging scans are obtained according to manufacturer specifications, the target is selected by using intraoperative images, and the miniframe is iteratively adjusted until the cannula is aimed at target with less than 1-mm projected radial error. A ceramic stylet and peel-away sheath are then placed to target, and the actual error is measured. If stylet placement is acceptable (as determined by the surgeon, with 1.5-mm radial error being the usual cutoff), the stylet is replaced by the DBS electrode, and the peel-away sheath is removed. Postplacement images may be performed immediately after electrode placement, with the proximal ends of the electrodes extended within the bore of the scanner (“externalized”), after the electrodes have been secured with silicone boots and coiled under the closed scalp incision (“internalized”), or both. Examples of iMRI are shown in Fig 1.
Sample iMRI before and after DBS placement. The left side of the figure shows intraoperative 3D T1 FLASH images of ceramic stylet placement in the left Vim (upper image, coronal; lower image, sagittal). The right side of the figure shows bilateral DBS electrode placement in the same patient. See “Surgical Procedure” in the Methods section for details.
Intraoperative and Postoperative Imaging
Surgeries were performed in a 1.5T, 70-cm bore MR imaging scanner (Aera; Siemens) using a vendor-supplied receive-only flexible coil (4 channels; 516 × 224 mm) outside of the sterile field. All imaging sequences performed after electrode placement used low-specific absorption rate (SAR)/B1+rms protocols (Table 1), keeping B1+rms below 2 µT and under 30 minutes of scan time, in accordance with the Infinity directions for use.9 In all cases, after skin closure, volumetric T1 imaging was performed for electrode localization, and most patients underwent FLAIR and DWI sequences to rule out vasogenic and cytotoxic edema. In selected cases, volumetric T1 imaging and additional target-specific sequences (eg, T2 for subthalamic nucleus [STN], white-matter-nulled [WMn] MPRAGE, or proton attenuation for globus pallidus internus [GPi]) were performed with externalized electrodes to confirm placement before skin closure.
MR imaging parameters
Postoperative CT scans were performed using the standard stereotactic protocol of 1-mm contiguous slices at zero gantry angle.
Data Analysis and Statistics
Data were analyzed by using Excel (Microsoft); descriptive statistics are reported.
Safety Determination and Consent
Before beginning the series, available MR imaging safety data (Papadaki and Thornton, unpublished data, 2016) for the Infinity electrodes were reviewed with our institutional MR Imaging Safety Committee, which granted permission to proceed by using manufacturer guidelines of low-SAR/B1+rms protocols after electrode placement. Standard surgical consent was obtained for all procedures, including the off-label nature of iMRI-guided DBS placement.
The University of Arizona Neuromodulation Clinical Data Base is maintained under the University of Arizona institutional review board #1906737419. No additional research informed consent was required because of the retrospective nature of the analysis. All research was performed according to the Declaration of Helsinki.
RESULTS
Patient and Electrode Characteristics
Thirty-four consecutive patients were identified. Patient characteristics, imaging data, and complications are summarized in Table 2. Median age was 69 years (range, 55–85 years). Twenty-three (68%) were male, likely reflecting our referral source from a local Veterans Administration hospital. Eighteen (53%) patients had PD, 14 (41%) ET, and 2 (6%) dystonia. There were 29 (85%) bilateral and 5 unilateral (2 left, 3 right) placements, for a total of 63 electrodes. Twenty-eight (44%) electrodes were placed in the STN, 26 (41%) in the nucleus ventralis intermedius (Vim), and 9 (14%) in the GPi. Fifty-nine electrodes (94%) had 0.5-mm contact spacing (Infinity Model 6172), and 4 electrodes (6%) had 1.5-mm spacing (Infinity Model 6173). Surgical time was typically 5–6 hours.
Patient characteristics
Stereotactic Accuracy
All electrodes were placed with a single pass. Accuracy (as measured at the tip of the ceramic stylet) was excellent, as previously published by using this technique. On the left, medial-lateral error was 0.3 ± 0.3 mm, anteroposterior error was 0.3 ± 0.2 mm, and radial error was 0.5 ± 0.3 mm. On the right, medial-lateral error was 0.3 ± 0.3 mm, anteroposterior error was 0.3 ± 0.3 mm, and radial error was 0.6 ± 0.3 mm.
iMRI after Electrode Placement
Individual patient imaging is listed in Table 3. A total of 4 patients underwent imaging with externalized electrodes before skin closure. After electrode internalization and skin closure, all 34 patients underwent volumetric T1 imaging. DWI and FLAIR sequences were performed in 33 (97%) and 27 (74%) patients, respectively. Twenty-four (71%) patients underwent additional target-specific sequences. The median number of postplacement sequences was 4 (range, 3–7). Mean ± SD total postplacement scan time was 10.5 ± 4.2 minutes (range, 5.6–24.3). Variability in the number of postplacement sequences per patient is explained by the use of different target-specific sequences in some cases and in the standardization of our postplacement protocol partway through the series.
MR imaging sequences obtained per patient after DBS placement
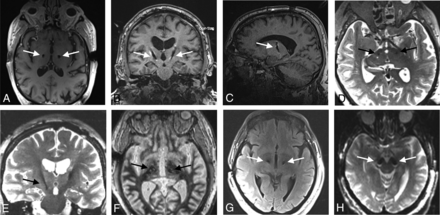
Imaging artifacts, although not a specific object of analysis in this study, were noted to be large, as is common with DBS electrodes under MR imaging. These were not used to determine stereotactic accuracy. Examples of electrode artifacts are shown in Fig 2.
Examples of the MRI artifacts of implanted Infinity DBS electrodes. The largest artifacts were seen in T1 images (A–C) and the smallest artifacts on T2 images (D, E). Artifacts on WMn (F), FLAIR (G), and DWI (H) sequences are also shown. Arrows indicate electrodes.
Complications
Intraoperative MR imaging showed intracerebral hemorrhage in 3 patients (9%). The first (patient 2) was a 6-mm cortical intracerebral hemorrhage that appeared after burr-hole placement before the dura was opened. The second (patient 8) was minimal subcortical hemorrhage and edema that appeared between stylet placement and electrode placement. Both of these were asymptomatic and required no intervention. The third (patient 20) was a 3-cm subcortical intracerebral hemorrhage that occurred during dural opening and coagulation of a bleeding surface vessel before any device placement. This was evacuated during the procedure and was asymptomatic. In summary, all 3 intraprocedural hemorrhages were small, asymptomatic, and related to surgical access itself rather than any identifiable interaction between the DBS electrodes and the MR imaging field.
On FLAIR images, 1 patient (2.9%) had mild insertional vasogenic edema. DWI in all cases was negative for acute ischemic change.
In patients with MR imaging both before and after scalp closure (n = 4), there was no evidence of electrode migration between scans or local heating.
Thirty of 34 patients (88%) had a postoperative CT within 24 hours of electrode placement. In addition to the expected evolution of the MR imaging findings mentioned, 2 patients had new findings. In 1 patient (patient 28), a new 7-mm subdural hematoma was seen adjacent to a burr-hole. This was asymptomatic and required no intervention. A second patient (patient 30) developed mild subcortical edema around the left electrode. This also was asymptomatic and required no intervention.
During the 30 days after surgery, no patients complained of scalp heating or showed evidence of heat-related injuries or postoperative neurologic deficit. Electrode impedance at the time of pulse generator implantation (within 2 weeks after electrode placement) was normal in all cases.
There was 1 postoperative death: patient 8 died from a small-bowel obstruction at 5 days after surgery. There was 1 serious complication: patient 33 developed intracerebral infection, seizure, and hemiparesis, requiring electrode removal and surgical washout on postoperative day 9, after which he made a full recovery. There were 4 minor complications: patient 10 developed a urinary tract infection, patient 11 developed mild self-limited confusion, patient 28 had a superficial stitch abscess treated with oral antibiotics, and patient 32 had hyponatremia treated with fluid restriction.
DISCUSSION
We present 34 consecutive cases of iMRI-DBS using Infinity electrodes. When using a low-SAR/B1+rms protocol for imaging after electrode placement, no apparent iMRI-related complications, such as heating, lesioning, edema, electrode movement, or damage to the electrodes, occurred. This mirrors prior ClearPoint experience with Medtronic electrodes.2⇓⇓-5
We found no difficulty in adhering to manufacturer guidelines for electrode imaging with any postplacement sequences. T1-weighted sequences and EPI inherently have a low SAR because of small flip angles and large TR and were easily adapted by using the low-SAR radiofrequency option. Sequences with the highest SAR (T2 and FLAIR) because of the high flip angles were addressed adequately by using the low-SAR radiofrequency pulse option and by increasing the TR and reducing the refocusing flip angle to 120 to obtain B1+rms below 2 μT.
Manufacturer guidelines now use B1+rms limits instead of SAR. The advantage of using B1+rms over SAR is that it is not patient-dependent. The protocol parameters affecting B1+rms, such as TR and flip angles, were unchanged after optimization on the first patient, so there was no variability across patients. The only consequence of sequence adjustment from our standard institutional protocols was increased scan time because of longer TR; however, because the patients were anesthetized, motion artifacts were not an issue.10
There were 5 identifiable intraprocedural complications: 3 intracranial hemorrhages, 1 subdural hematoma, and 1 case of electrode-related edema, among 34 patients and 63 electrodes. None of these complications appeared to have any relationship to the iMRI environment or postplacement imaging of the electrodes but were all related to cranial access or ceramic stylet placement. Although our overall hemorrhage rate (11.7% per procedure, 6.3% per electrode) was higher than in previously published large iMRI series,5 all hemorrhages were small, asymptomatic, and clinically insignificant. Postoperative complications were, similarly, all well-known surgical complications and showed no apparent relationship to the iMRI environment or postplacement iMRI scans.
In the time since we began iMRI-DBS using Infinity electrodes, Abbott has received FDA approval for MR imaging scanning of the internalized “leads-only” configuration.9 However, we believe our results are still useful for several reasons. First, we additionally demonstrate safety of imaging with externalized electrodes. Second, postmarket safety data have not yet been published. Third, our patients are under anesthesia, providing reassurance that patient feedback is not necessary to ensure safe imaging. Fourth, we provide evidence for the safety of the iMRI-DBS approach using directional electrodes in general. The programing flexibility permitted by directional electrodes combined with the accuracy of iMRI guidance allows a high degree of confidence in electrode placement even for targets with little immediate physiologic feedback, such as targets for psychiatric diseases, and may allow real-time biomarker assessment such as changes in functional MR imaging during electrode placement.11,12
Limitations of this study include the inability to report transient events such as scalp warming, motor activity, or paresthesias. These may emerge from the ongoing Abbott postmarket study,6 which will be conducted in awake patients; however, transient sensorimotor effects would have no significance for patients undergoing iMRI-DBS under general anesthesia. Second, this study could be underpowered for low-frequency events. Finally, our results reflect the use of a single MR imaging scanner, though there would be no reason to suspect that the results would be different with other MR imaging models or manufacturers as long as manufacturer guidelines for B1+rms and scan time are similarly followed.
CONCLUSIONS
We present real-world data on the safety of Infinity DBS placement in an interventional MR imaging suite. When manufacturer guidelines for MR imaging safety by using fully implanted leads are followed, there appear to be no MR imaging–related safety issues with this technique. These data should be useful for other institutions considering iMRI-DBS placement using these devices.
Acknowledgments
The authors thank Janette Zingg and Sarah Cyr for assistance in collecting MR imaging parameters. The senior author is a site principal investigator for the Abbott Infinity postmarket MR imaging safety study,6 for which no data are yet available and for which he receives no compensation. No patients reported here will be duplicated in the Abbott-sponsored study.
Footnotes
Disclosures: Jennifer Becker—UNRELATED: Consultancy: Nuvox Pharma and Siemens Syngo Via, Comments: Siemens-Consultancy, money paid to department, not related to this article, Nuvox-Consultancy work at AHA meeting 2019 Nuvox, not related to study, honorarium paid to me with travel expenses reimbursed*; Payment for Lectures Including Service on Speakers Bureaus: Canon (Vitrea), Comments: Lecture given on behalf of Canon, Phoenix 2020.* *Money paid to the institution.
References
- Received April 28, 2020.
- Accepted after revision June 15, 2020.
- © 2020 by American Journal of Neuroradiology