Abstract
BACKGROUND AND PURPOSE: Knowledge about predictors of the outcome of flow-diverter treatment is limited. The aim of this study was to predict the angiographic occlusion status after flow-diverter treatment with computational fluid dynamics using porous media modeling for decision-making in the treatment of large wide-neck aneurysms.
MATERIALS AND METHODS: A total of 27 patients treated with flow-diverter stents were retrospectively analyzed through computational fluid dynamics using pretreatment patient-specific 3D rotational angiography. These patients were classified into no-filling and contrast-filling groups based on the O’Kelly-Marotta scale. The patient characteristics, morphologic variables, and hemodynamic parameters were evaluated for understanding the outcomes of the flow-diverter treatment.
RESULTS: The patient characteristics and morphologic variables were similar between the 2 groups. Flow velocity, wall shear stress, shear rate, modified aneurysmal inflow rate coefficient, and residual flow volume were significantly lower in the no-filling group. A novel parameter, called the normalized residual flow volume, was developed and defined as the residual flow volume normalized by the dome volume. The receiver operating characteristic curve analyses demonstrated that the normalized residual flow volume with an average flow velocity of ≥8.0 cm/s in the aneurysmal dome was the most effective in predicting the flow-diverter treatment outcomes.
CONCLUSIONS: It was established in this study that the hemodynamic parameters could predict the angiographic occlusion status after flow-diverter treatment.
ABBREVIATIONS:
- AIRC
- aneurysmal inflow rate coefficient
- CF
- contrast-filling
- CFD
- computational fluid dynamics
- FD
- flow diverter
- FV
- flow velocity
- mAIRC
- modified aneurysmal inflow rate coefficient
- NF
- no-filling
- nRFV
- normalized residual flow volume
- RFV
- residual flow volume
- SR
- shear rate
- WSS
- wall shear stress
Recanalization and retreatment are frequent even after using stent-assisted coil embolization for large wide-neck internal carotid artery aneurysms. The safety and efficacy of the flow diverter (FD) treatment1,2 have been recently demonstrated in many studies. In the large-scale Pipeline for Uncoilable or Failed Aneurysms study, approximately 76% of the patients had complete aneurysm occlusion on 6-month angiography and 93% had complete aneurysm occlusion on 3-year angiography after treatment.1,2
In contrast, some aneurysms need additional treatment and may otherwise rupture. Delayed aneurysm rupture and intraparenchymal hemorrhages are poorly understood and usually lead to fatal complications.3,4 Rouchaud et al4 reported that 81.3% of patients with delayed ruptures died or had poor neurologic outcomes and 76.6% of the delayed ruptures occurred within 1 month posttreatment. However, predicting whether an aneurysm would be completely occluded or ruptured after the FD treatment is difficult.
In recent times, the development of computational fluid dynamics (CFD) has enabled the evaluation of hemodynamics of cerebral aneurysms. Umeda et al5,6 reported the hemodynamic characteristics required to predict the recurrence of coiled aneurysms with CFD using porous media modeling, which can be effective in predicting the postcoiling aneurysm occlusion status. Application of the porous media setting to an intracranial stent may also aid in simulating the hemodynamics after the endovascular treatment with an FD stent.7
The aim of this study was to evaluate the effectiveness of porous media CFD for predicting the angiographic occlusion status after FD treatment.
MATERIALS AND METHODS
This retrospective study was approved by the institutional ethics committee. The patients provided written informed consent for using their data in this study.
Patient Population and Study Protocol
During 2015–2017, thirty-four unruptured internal carotid artery aneurysms were treated with FD using a Pipeline Embolization Device (Covidien) after 3D rotational angiography.
We evaluated the angiographic occlusion status during follow-up DSA at 6–12 months postprocedure. The DSA findings were classified into 2 groups using the O’Kelly-Marotta classification.8 We classified the O’Kelly-Marotta classes A (A1, A2, A3), B (B1, B2, B3), and C (C1, C2, C3) as the contrast-filling (CF) group and D as the no-filling (NF) group. Two independent observers who were blinded to the hemodynamic results evaluated and classified the O’Kelly-Marotta grading.
Investigators who were unaware of the occlusion status performed CFD analyses independently. We compared the morphologic variables and hemodynamic parameters of the CF group with those of the NF group. We also evaluated the effectiveness of the CFD analysis for predicting the angiographic occlusion status after FD treatment.
Morphologic Variables
As previously described, the morphologic variables were measured on stereolithographic models generated from preoperative 3D rotational angiography using ImageJ (National Institutes of Health) and CFX-post (CFX CFD19.2; ANSYS).
CFD Analysis
The patient-specific geometries were generated from the DICOM datasets of preoperative 3D rotational angiography that were obtained using Artis zee dBA Twin (Siemens). The surface reconstruction of the computational model was obtained by segmenting the lumen boundary from 3D rotational angiography images using an analytic software (Mimics 16.0; Materialise). The stereolithography was re-meshed to improve the surface triangle quality (3-matic 8.0; Materialise). The 3D neck part was created using a Boolean subtraction between the original geometry and aneurysm-deleted models. A 3D stent domain was obtained by a transformation, in which the 3D neck part was offset by a thickness of 0.048 mm, which corresponded to the diameter of the strut of the Pipeline. The computational hybrid meshes were generated with tetrahedral and prism elements (ICEM CFD19.2; ANSYS). The size of the tetrahedral elements was 0.1–1.2 mm for the fluid domain and 0.05 mm for 3D stent domain. Five prismatic boundary layers with a total thickness of 0.15 mm covered the surface to ensure an accurate definition of the velocity gradient. The average mesh number we used in this study was about 3.8 million. Volumetric meshes were used to ensure sufficient mesh resolution around the 3D stent domain. The effect of mesh density on 3D stent domain controls (the mesh independence study) showed that the mesh size did not remarkably affect hemodynamic parameters evaluated in this study. A straight inlet extension was added to the inlet section to obtain a completely developed laminar flow.
For the fluid domain, the 3D incompressible laminar flow fields were obtained by solving the continuity and Navier–Stokes equations. Numeric modeling was performed using a commercially available CFD package (CFX 19.2; ANSYS). Blood was assumed to be an incompressible Newtonian fluid with a blood density of 1056 kg/m3 and a blood dynamic viscosity of 0.0035 Pa × s. The typical flow waveform of phase-contrast MR imaging was scaled to achieve the physiologic wall shear stress (WSS).9,10
Traction-free boundary conditions were applied at all outlets. When there were several outlets, we set the outlets at a certain distance and height from the aneurysm to prevent uneven distribution of blood flow. The steady-state analysis was performed using the mean flow-volume rate. We used the typical flow waveform of phase-contrast MR imaging to achieve the physiologic WSS based on the Murray law. We used the blood flow rate set by the vessel diameter of the inlet. Thus, the steady flow analysis was assumed to use the mean flow-volume rate.
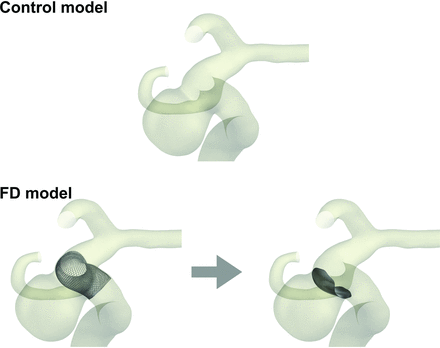
We conducted the 2-pattern CFD simulations, which involved the simulation of an untreated stent-free aneurysm as the preoperative status (control model) and assuming an aneurysm with a simple placement of an FD stent (FD model) (Fig 1).
Images of porous media modeling for FD treatment. The control model enables the simulation of preoperative status, whereas the FD model enables the simulation of simple deployment of an FD stent.
FD Model
In this study, we applied the 2 numeric models in the fluid and porous domains. The flow in the fluid domain was simulated using the Navier–Stokes equation and equation of continuity as follows:

 where v is the velocity of the flow, p is the pressure, ρ is the density, μ is the viscosity of the fluid, and F is the body force.
where v is the velocity of the flow, p is the pressure, ρ is the density, μ is the viscosity of the fluid, and F is the body force.
The flow in the stent regimes was simulated using porous media modeling that follows the Darcy law. In these flow regimes, pressure was locally balanced with resistance forces, such that
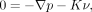 where K is a constant of porous resistance. This was assumed to be a quasilinear function of the magnitude of velocity,
where K is a constant of porous resistance. This was assumed to be a quasilinear function of the magnitude of velocity,
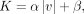 where α and β are coefficients, for which the values are determined using the Ergun equation as follows:
where α and β are coefficients, for which the values are determined using the Ergun equation as follows:

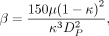 where DP is the average particle diameter.11 It was set to 0.048 mm, which corresponded to the diameter of the strut of the Pipeline.
where DP is the average particle diameter.11 It was set to 0.048 mm, which corresponded to the diameter of the strut of the Pipeline.
The volume coverage ratio is defined as the ratio of the volume of the stent strut to the stent volume. The variable κ12 denotes the porosity of the porous media stent.

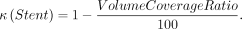
The metal coverage ratio is defined as the ratio of the area of the stent strut to the stent area. The relationship between the volume coverage ratio and metal coverage ratio is defined using the following formula:
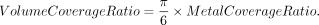
The metal coverage ratio of the Pipeline was considered to be approximately 30%. Thus, we estimated the volume coverage ratio as 15%. In an aneurysm treated by overlapping 2 FDs, the volume coverage ratio was assumed to be 30% for aneurysmal flow reduction. To simulate the hemodynamics in an occlusion status, we calculated hemodynamic parameters under steady-state conditions. The aneurysmal inflow rate coefficient (AIRC) was used as a predictor of recanalization after coil embolization in basilar tip aneurysms.13 The original AIRC is the proportion of the aneurysmal inflow rate to the basilar artery flow rate. We modified this parameter for internal carotid artery aneurysms, and the modified AIRC (mAIRC) denoted the proportion of the aneurysmal inflow rate to the proximal parent artery flow rate.
The residual flow volume (RFV) was calculated to predict the postcoiling angiographic results in unruptured cerebral aneurysms.5,6 The RFV was the volume of fluid domain in the aneurysmal dome and was defined as the volume with more than the following mean flow velocity (FV): Tested thresholds included 4, 6, 8, 10, 12, 14, 16, 18, and 20 cm/s. Because RFV was affected by the volume of aneurysmal dome, we developed a novel parameter, the normalized residual flow volume (nRFV), which is defined as RFV normalized by the dome volume. The nRFVα denoted nRFV with an average FV of over α cm/s. We calculated the mAIRC in the control model, RFV in the FD model, and FV, WSS, and shear rate (SR) in both models.
Statistical Analysis
All values were expressed as mean ± SD. Statistical analyses were performed using the software environment R (Version 3.3.3; http://www.r-project.org/). We compared the variables between the CF and NF groups using the Welch t test. P values < .05 were considered statistically significant, while the area under the receiver operating characteristic curve was used to determine the diagnostic accuracy of predicting intra-aneurysmal thrombosis.
RESULTS
Among the 34 patients, 7 were excluded because of the difficulty in reconstructing the vascular geometry from 3D rotational angiography. Therefore, 27 patients with 27 unruptured aneurysms, which were treated endovascularly with an FD stent, fulfilled the criteria and constituted the study population. The patients’ characteristics, aneurysm characteristics, and treatment characteristics in the 2 groups are summarized in Table 1.
Comparison of patient characteristicsa
Differences between the NF and CF Groups on Univariate Analyses
Patient Characteristics and Aneurysmal Morphologic Variables.
No significant differences were observed in terms of patient characteristics and aneurysmal morphologic variables between the NF and CF groups (Tables 1 and 2).
Comparison of morphologic variablesa
Aneurysmal Hemodynamic Factors.
Among the hemodynamic parameters in the control CFD, FV (0.16 m/s versus 0.28 m/s; P = .003), WSS (2.92 Pa versus 4.66 Pa; P = .03), SR (8.12 × 102 1/s versus 12.83 × 102 1/s; P = .03), and the mAIRC (0.14 versus 0.29; P = .04) were significantly lower in the NF group (Table 3).
Comparison of hemodynamic parametersa
The porous media CFD showed that FV (0.10 m/s versus 0.16 m/s; P = .002), WSS (0.67 Pa versus 1.00 Pa; P = .03), SR (7.22 × 102 1/s versus 10.55 × 102 1/s; P = .03), RFV14 (140.1 mm3 versus 329.7 mm3; P = .02), RFV16 (110.2 mm3 versus 250.6 mm3; P = .02), RFV18 (84.4 mm3 versus 181.5 mm3; P = .03), and RFV20 (58.3 mm3 versus 142.9 mm3; P = .04) were significantly lower in the NF group than in the CF group (Tables 3 and 4). Similar findings were observed for nRFV6 (0.77 versus 0.95; P = .02), nRFV8 (0.64 versus 0.92; P = .004), nRFV10 (0.54 versus 0.88; P = .002), nRFV12 (0.46 versus 0.82; P = .001), nRFV14 (0.37 versus 0.74; P = .001), nRFV16 (0.29 versus 0.64; P = .004), nRFV18 (0.24 versus 0.53; P = .02), and nRFV20 (0.17 versus 0.43; P = .03) (Table 4).
Comparison of RFV and nRFVa
Receiver Operating Characteristic Curve Analyses of Possible Determinants for Intra-Aneurysmal Thrombosis after FD Treatment
Among the parameters that demonstrated significant differences between the CF and NF groups on univariate analyses, nRFV8 demonstrated the highest area under the receiver operating characteristic curve value of 0.85 (95% CI, 0.71–1.00). The cutoff value was 0.93 with a sensitivity of 0.88 and a specificity of 0.73 (Tables 5 and 6).
ROC curve analysis for hemodynamic parameters that had significant differences between the CF and NF groups on univariate analyses to predict intra-aneurysmal thrombosis after FD treatment
ROC curve analysis for RFV and nRFV that had significant differences between the CF and NF groups on univariate analyses to predict intra-aneurysmal thrombosis after FD treatment
Representative Cases
Case 1: NF.
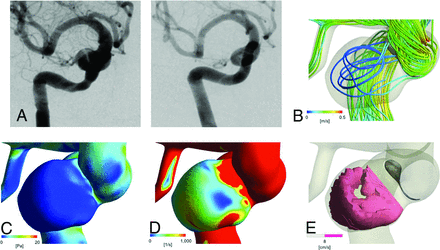
A 58-year-old woman had a left cavernous portion aneurysm (Fig 2). The maximum size and neck width were 10.1 and 7.7 mm, respectively. In the control model, FV, WSS, SR, and the mAIRC were 0.08 m/s, 1.13 Pa, 3.20 × 102 1/s, and 0.02, respectively. In the FD model, FV, WSS, and SR were 0.06 m/s, 0.37 Pa, and 3.96 × 102 1/s, respectively. RFV16 and nRFV8 were 11.1 mm3 and 0.38, respectively. We treated this aneurysm with FD using a Pipeline Embolization Device (4.5 × 20 mm). DSA at 6 months after treatment showed complete disappearance of the aneurysm.
Representative no-filling case. A 58-year-old woman with an asymptomatic aneurysm. A, Digital subtraction angiography shows a large wide-neck aneurysm located at the left cavernous portion of the internal carotid artery (left), which completely disappeared 6 months after the FD treatment (right). B, Streamline of FV in the FD model. The mean FV at the dome is 0.06 m/s. C, Contour line of wall shear stress in the FD model (mean value at the dome is 0.37 Pa). D, Contour line of the shear rate in the FD model (mean value at the dome is 3.96 ×102 1/s). E, Intra-aneurysmal nRFV of 0.38 with an average flow velocity of >8 cm/s.
Case 2: CF.
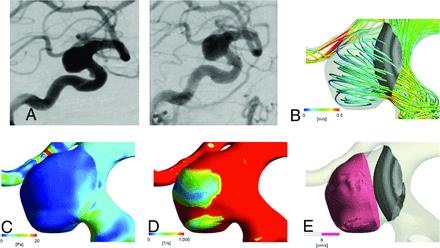
A 73-year-old woman had a left paraclinoid-portion aneurysm (Fig 3). The maximum size and the neck width were 12.4 and 11.7 mm, respectively. In the control model, the values of FV, WSS, SR, and the mAIRC were 0.43 m/s, 7.89 Pa, 21.68 × 102 1/s, and 0.54, respectively. In the FD model, FV, WSS, and SR were 0.22 m/s, 1.25 Pa, and 12.8 × 102 1/s, respectively. RFV16 and nRFV8 were 429.0 mm3 and 0.96, respectively. We treated this aneurysm with a FD using a Pipeline Embolization Device (4.75 × 25 mm). The DSA at 6 months after treatment showed an incomplete disappearance of the aneurysm.
Representative contrast-filling case. A 73-year-old woman with an asymptomatic aneurysm. A, Digital subtraction angiography shows a large wide-neck aneurysm located at the left paraclinoid portion of internal carotid artery (left), which does not disappear at 6 months after the FD treatment (right). B, Streamline of FV in the FD model. The mean FV at the dome is 0.22 m/s. C, Contour line of wall shear stress in the FD model (mean value in the dome is 1.25 Pa). D, Contour line of the shear rate in the FD model (mean value in the dome is 12.8 × 102 1/s). E, Intra-aneurysmal nRFV of 0.96 with an average flow velocity of >8 cm/s.
DISCUSSION
In this study, we demonstrated that the hemodynamic parameters using both control CFD and porous media CFD could predict the angiographic occlusion status at 6 months after the FD treatment. In addition, nRFV8 was the strongest predictor. These results indicated that preoperative CFD could be effective in decision-making during the treatment of large wide-neck internal carotid artery aneurysms.
A previous CFD study with high-fidelity virtual stent placement showed that posttreatment aneurysmal flow reduction, including the average FV, WSS, inflow rate, and turnover time, could be correlated with aneurysmal occlusion outcomes.14 In the in vivo studies, local FV and SR were significantly smaller and the mean transit time was higher in occluded aneurysms than in the rest of the cases.15 These results suggested that hemodynamic reduction in terms of FV, SR, and WSS is the most important mechanism to induce intra-aneurysmal thrombosis. Corbett et al16 reported that flow‐induced thrombosis occurred under an SR threshold of 54 seconds−1 or a specific WSS threshold of 0.41 Pa. These in vivo and in vitro results support the findings of the present study.
We also demonstrated that the NF group had significantly lower mAIRC and RFV values. These results were similar to those reported by Sugiyama et al13 and Umeda et al.5,6 Sugiyama et al stated that the AIRC was an independent and significant predictor for recanalization after coil embolization. Umeda et al reported that RFV was an effective parameter in predicting the recurrence of coiled aneurysms. It was suggested that mAIRC and RFV could be good predictors for the angiographic occlusion status after the FD treatment, as in the case of coil embolization.5,6,13 In this study, the receiver operating characteristic curve analyses demonstrated that nRFV8.0 had the strongest predictive capability among all studied parameters.
The clinical implication of the present study is the ability to predict whether the FD treatment is effective for every individual case. Mut et al17 demonstrated that the aneurysms in the fast occlusion group demonstrated a lower mean FV, inflow rate, and SR. However, these values were determined under posttreatment hemodynamic conditions. Therefore, it remains unknown whether their findings could be used for treatment decision-making. In contrast, the FV at an aneurysm dome using a porous media setting can simulate the outcomes of selected reconstructive treatments, such as direct operations with respect to trapping of an aneurysm and parent artery with high-flow bypass and FD treatment with/without coils.
To treat an aneurysm with insufficient FV reduction by endovascular treatment, we considered the following 2 treatments to increase the reduction of FV. The first approach involves FD stent placement with coils. In a previous study, it was demonstrated that the FD treatment with coils further reduced FV and WSS and increased the low wall shear area.18 Double-porous media CFD can be used to predict the posttreatment angiographic aneurysmal occlusion status preoperatively.19 The other approach involves overlapping FD stents or performing compacted FD stent placement. Some reports showed that overlapped or compacted FD stents reduced the values of hemodynamic parameters, including the mean intra-aneurysmal FV, WSS, and inflow rate.20 Therefore, FD stent placement with coils, overlapped FD stent placement, and compacted FD stent placement can be considered as treatment options for aneurysms with insufficient FV reduction.
The present study has several limitations. First, this study is simple and retrospective. A prospective larger cohort study that includes all types of aneurysms is required to establish the effectiveness of hemodynamic simulation for predicting the outcome of the FD treatment. Second, all the patients who participated in the study were Japanese. Some studies indicated that there was a racial difference in platelet function.21 They suggested that ethnicity might be an important factor in determining the outcome of the FD treatment. Third, other factors could influence the outcome of the FD treatment, including body forces such as blood viscosity and blood pressure, antiplatelet effect, aneurysm location, branching artery, and incomplete FD expansion. It is difficult for this model to accurately reflect the stent-placement conditions that can influence the hemodynamic factors, but this model makes a distribution of stent uniform.
In addition, we cannot overlook the limitations of the CFD studies. The vessel walls were assumed to be rigid, and blood was modeled as a Newtonian fluid in this study. Newtonian fluids may overestimate WSS and SR at the aneurysmal dome.22 Therefore, our quantitative CFD results might have some bias. Further studies are thus necessary to validate the results under different simulations.
CONCLUSIONS
All studied hemodynamic factors were significantly lower in the NF group, and nRFV8 was observed to be the strongest predictor of aneurysmal occlusion status after the FD treatment. It was shown that CFD could be useful in pretreatment planning, thereby contributing to more reliable and effective FD treatments for cerebral aneurysms.
Acknowledgments
We thank Editage (www.editage.jp) for English language review of the manuscript.
Footnotes
Disclosures: Manabu Shirakawa—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Stryker, Comments: lecture fee. Shinichi Yoshimura—RELATED: Consulting Fee or Honorarium: Medtronic, Comments: lecture fee; UNRELATED: Consultancy: Kaneka Medix; Payment for Lectures Including Service on Speakers Bureaus: Boehringer Ingelheim, Daiichi Sankyo, Bayer Yakuhin, Ltd. Bristol-Meyers Squibb, Stryker, Sanofi, Otsuka Pharmaceutical, Johnson & Johnson, MicroVention Terumo, Biomedical Solutions.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 11, 2020.
- Accepted after revision July 7, 2020.
- © 2020 by American Journal of Neuroradiology