Abstract
BACKGROUND AND PURPOSE: White matter hyperintensities on T2-weighted MR imaging are typical in older adults and have been linked to several poor health outcomes, including cognitive impairment and Alzheimer disease. The presence and severity of white matter hyperintensities have traditionally been attributed to occlusive arteriopathy, but recent evidence also implicates deep medullary venule collagenosis and associated vasogenic edema. Historically, postmortem analyses have been the sole way to analyze cerebral veins, but SWI can be now used to examine cortical veins in vivo. The aim of the current study was to determine whether there is an association between the diameters of the large draining cerebral veins/sinuses and white matter hyperintensity volume.
MATERIALS AND METHODS: T2-weighted FLAIR and SWI were performed in 682 older adults without dementia (mean age, 73.9 ± 5.9 years; 59.1% women). Total and regional white matter hyperintensity volume was derived. We measured the diameters of 5 regions of the cerebral venous draining system: internal cerebral veins, basal veins of Rosenthal, superior sagittal sinus, vein of Galen, and straight sinus terminus.
RESULTS: Increased diameter of the internal cerebral veins was associated with greater total white matter hyperintensity volume (β = 0.09, P = .02) and regionally in the parietal (β = 0.10, P = .006), frontal (β = 0.09, P = .02), and temporal (β = 0.09, P = .02) lobes. Increased diameter of the basal veins of Rosenthal was associated with greater total (β = 0.10, P = .01), frontal (β = 0.11, P = .003), and temporal (β = 0.09, P = .02) white matter hyperintensity volume.
CONCLUSIONS: Our results suggest that the caliber of the internal cerebral veins and of the basal veins of Rosenthal relates to regional white matter disease.
ABBREVIATIONS:
- AD
- Alzheimer disease
- WHICAP
- Washington Heights–Inwood Columbia Aging Project
- WMH
- white matter hyperintensity
- ICC
- intraclass correlation coefficient
Chronic ischemia of the white matter of the brain can result in demyelination and axonal loss, manifesting primarily as white matter hyperintensities (WMHs), or “leukoaraiosis,” on T2-weighted MR imaging.1,2 Increased WMH volume is associated with a number of suboptimal health outcomes, including cognitive decline, elevated Alzheimer disease (AD) risk, AD-related genetic profiles, bipolar disorder, and stroke risk.3⇓⇓⇓⇓–8
The importance of vascular dysfunction and AD is increasingly appreciated, but the underlying mechanisms behind this relationship remain unclear.9 Although periventricular venous collagenosis was linked to WMHs > 20 years ago,10 the clinical observations associating vascular disease with WMHs typically assume that they are solely arterial in origin. The idea that venous pathology contributes to the radiologic manifestation of WMHs is now being revisited, and contemporary hypotheses suggest that the pathology of the veins and venules may at least partially mediate the association between WMHs and AD.11 Periventricular venous collagenosis and large-caliber venous stenosis may be 1 mechanism through which WMHs and AD are associated, and venous collagenosis may increase overall resistance in the veins, leading to reduced blood flow to the deep white matter.12 Venous outflow obstruction may also lead to decreased clearance of metabolic by-products and toxic misfolded proteins as seen in AD, resulting in ischemic stress.12
Cerebral veins have typically been distinguished from the arterial and capillary systems by postmortem analysis with complex immunostaining. Studies of postmortem tissue noted that concentric collagen deposition in the deep medullary venular system (eg, luminal stenosis or occlusion) correlates with WMH severity.10,13 Historically, the cortical veins have been difficult to distinguish on brain imaging, but SWI can detect cortical veins that are difficult to visualize on T2-weighted or proton density images. With this technique, venous vessels become hypointense due to the magnetic susceptibility differences between oxygenated and deoxygenated blood. Even without contrast administration, SWI is particularly sensitive to the visualization of periventricular veins.14 In this study, we used SWI to visualize and measure the diameters of large draining veins in the brain to test the hypothesis that cerebral basal vein dilation is related to WMH volume.
Materials and Methods
Study Subjects
The Washington Heights–Inwood Columbia Aging Project (WHICAP) is an ongoing epidemiologic study of cognitive aging and dementia in the racially/ethnically diverse community surrounding Columbia University Medical Center in upper Manhattan. It includes randomly sampled adults 65 years or older; there were no specific inclusion/exclusion criteria other than age. More than 6000 participants have been recruited into WHICAP since its inception in 1992. Neuropsychological, medical, neurologic, demographic, and psychosocial data are collected on all active participants at approximately 24-month intervals. Neuroimaging was incorporated into WHICAP in 2 waves, beginning in 2004 and in 2009. For the neuroimaging substudies, participants were enrolled who did not meet the diagnostic criteria for dementia at their previous or closest longitudinal visit. In the current study, we analyzed participants with MR imaging data from the second wave, which included 3T scans with T1-weighted, T2-weighted FLAIR, and SWI sequences. We ascertained vascular risk history based on chart review and participant interview and self-reported history of heart disease, hypertension, and type 2 diabetes. Each vascular risk factor was established by a diagnostic history and/or documentation or report of treatment for the condition, including medication or other medical intervention. These 3 dichotomous variables were added to create a vascular risk factor summary score that ranged from 0 to 3, as we have done in past studies.15⇓–17 The study was approved by the Columbia University Medical Center ethics committee; all participants gave written informed consent.
MR Imaging
MR imaging was performed using a 3T Intera scanner (Philips Healthcare, Best, the Netherlands). Images included T1-weighted (TR = 6.6 ms, TE = 3.0 ms, flip angle = 8°, FOV = 256 × 256 × 165 mm, section thickness =1.0 mm), T2-weighted FLAIR (TR = 8000 ms, TE = 337 ms, inversion recovery time = 2400 ms, flip angle = 90°, FOV = 240 × 240 × 180 mm, section thickness =2.0 mm), and SWI (TR = 16 ms, TE = 22 ms, flip angle = 15°, FOV = 512 × 512 × 150 mm, section thickness = 1.0 mm) sequences.
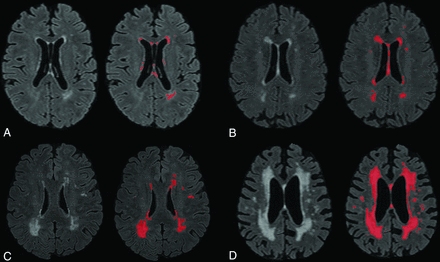
T1-weighted images were used to estimate total intracranial volume using FreeSurfer (Version 6.0; http://surfer.nmr.mgh.harvard.edu). FLAIR sequencing was used to derive total WMH volume using previously described techniques developed in our laboratory (Fig 1).18,19 In short, FLAIR images were skull-stripped and normalized, and a Gaussian curve was fit to map the voxel intensity values. If the voxels were >2.1 SDs above the mean FLAIR image, they were labeled as WMHs. This threshold of 2.1 SDs was selected on the basis of the greatest sensitivity and specificity of detection across participants, as determined by trained experts. White matter hyperintensity volume in cubic centimeters was calculated as the sum of labeled voxels multiplied by voxel dimensions. All labeled images were visually checked for errors, and manual corrections were made in the case of false-positive errors. The most common false-positive errors were small “speckles” of voxels labeled as hyperintense in the cortex, likely due to subtle intensity inhomogeneity that is common on T2-weighted images. The major lobes of the brain were derived by applying a standard spatial “lobar” atlas, and regional WMHs were defined as the intersection of labeled voxels and each segmented cerebral lobe.
Four sample images of WMH on T2 FLAIR, labeled for quantification using an intensity threshold with our software. A, Minimal WMHs. B, Mild WMHs. C, Moderate WMHs. D, Severe WMHs.
Vein and Sinus Measurements
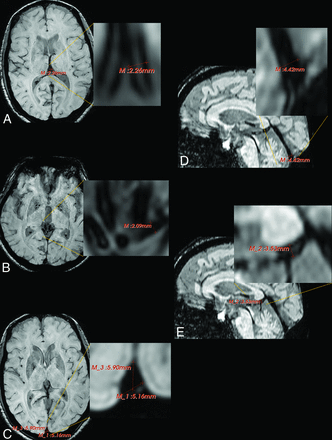
3D Slicer 4.8.1 (www.slicer.org) was used to measure the veins and sinuses on SWI (Fig 2).20 In the axial plane, the ruler function was used to measure the diameters of the left and right internal cerebral veins in millimeters. Due to variations in venous morphology and image quality, we visually identified the region of the vein that appeared most continuously linear and measured the diameter of the vein there. In each image, the measurements for the left and right internal cerebral veins were averaged for 1 value. Also, in the axial plane, the base and the anterior-posterior diameter of the superior sagittal sinus were measured immediately above the confluence of sinuses. These measurements were then averaged for 1 value. We also measured the widths of the left and right basal veins of Rosenthal in the axial plane just before they converged with the internal cerebral veins into the vein of Galen. These values were averaged for a singular measurement. In the sagittal plane, the vein of Galen was measured directly inferior to the splenium, where the width of the vein appeared most consistent. In addition, in the sagittal plane, the straight sinus was measured at its terminus immediately anterior to the confluence of sinuses. Intrarater reliability of all measurements was confirmed with intraclass correlation coefficients (ICCs; see the following section).
Sample depiction of vein/sinus measurement on SWI using 3D Slicer. A, The diameters of the left and right internal cerebral veins were measured in the axial plane and then averaged. B, The diameters of the left and right basal veins of Rosenthal were measured at their termini in the axial plane and then averaged. C, The base and anterior-posterior diameters of the superior sagittal plane were measured in the axial plane and then averaged. D, The straight sinus terminus was measured in the sagittal plane. E, The vein of Galen was measured inferior to the splenium in the sagittal plane.
Statistical Analyses
Descriptive statistics were generated for participant demographic data. To test for intrarater reliability of the vein and sinus measurements, we randomly selected 40 subjects and remeasured their veins and sinuses without knowledge of the previous measurement. Of the 10 forms of ICCs, a single-rating, absolute-agreement, 2-way random-effects model was chosen on the basis of recently described selection and reporting guidelines.21,22
Linear regression was used to test the primary hypotheses of this study. Each vein/sinus measurement was entered as the independent variable, and total WMH volume was set as the dependent variable. We performed another linear regression with the same variables, adjusting for age at the time of the scan, estimated intracranial volume, and the aforementioned vascular risk factor score. These linear models were run again separately for each cerebral lobe (frontal, temporal, parietal, and occipital) as dependent variables. After that, all regression analyses were repeated separately for all of the measured vein/sinus diameters. Z scores were also derived for all variables, and these values were used to calculate confidence intervals for the standardized β values.
Results
Sample Characteristics
Six hundred eighty-two participants from the WHICAP study had complete SWI, WMH, and demographic data. Participants had a mean age at scanning of 73.9 ± 5.9 years, and 59.1% were women. There was a fairly even distribution of race/ethnicity among whites, blacks, and Hispanics. The median time interval between the closest clinical follow-up visit and MR imaging was 38 days. Complete demographic data, including vascular disease and primary MR imaging measurements, are shown in Table 1.
Study sample characteristics and mean values for measured vein/sinus diameters and WMH volume
Intrarater Reliability
All 5 measurements had ICC values indicating either moderate or good reliability (internal cerebral veins: 0.81 [95% CI, 0.67–0.90], basal veins of Rosenthal: 0.73 [95% CI, 0.54–0.85], superior sagittal sinus: 0.68 [95% CI, 0.37–0.84], vein of Galen: 0.76 [95% CI, 0.50–0.88], and straight sinus: 0.76 [95% CI, 0.60–0.87]).22
Relationship between Vein/Sinus Measurements and WMH Volumes
In the unadjusted model, increased diameters of the internal cerebral vein, basal veins of Rosenthal, and the superior sagittal sinus were associated with higher total WMH volume (Table 2). Regionally, the internal cerebral vein diameter was associated with greater WMH volume across all brain lobes; the basal veins of Rosenthal were associated with greater WMH volume in the frontal, temporal, and parietal lobes; and the superior sagittal sinus was associated with greater WMH volume in the temporal, parietal, and occipital lobes.
Unadjusted associations (using standardized β values and corresponding 95% CI) between sinus/vein diameters and WMH volumea
When we controlled for age, estimated intracranial volume, and vascular risk factors in the adjusted model, the association between the internal cerebral veins and occipital lobe WMH volume was attenuated, the association between the basal veins of Rosenthal and parietal lobe WMH volume was attenuated, and all associations pertaining to the superior sagittal sinus were attenuated (Table 3). We found that the increased diameter of the internal cerebral veins maintained associations with higher total, frontal, temporal, and parietal WMH volume. An increased diameter of the basal veins of Rosenthal maintained associations with higher total, frontal, and temporal WMH volume. The diameters of the vein of Galen and straight sinus terminus were not associated with WMH volume in any of our analyses, either globally or regionally.
Associations (using standardized β values and corresponding 95% CI) between sinus/vein diameters and WMH volume after adjusting for age, estimated intracranial volume, and vascular risk factors
Discussion
Strong evidence links cerebrovascular disease with WMH. Although current models do not typically implicate veins or venules in the development of WMH, recent data suggest that they may play a role.11 In this cross-sectional study of older adults, we hypothesized that increased venous diameter would be associated with increased WMH volume. We found that increased diameters of the internal cerebral veins and the basal veins of Rosenthal were associated with greater WMH volume (Table 3). The strongest association for the internal cerebral veins was in the parietal lobe, the brain region where past studies have most consistently identified a correlation between WMH and AD.23 The strongest association for the basal veins of Rosenthal was in the frontal lobe.
Our study builds on past studies that identified a relationship between periventricular venous collagenosis and WMHs.10,12 In the first description of this phenomenon >20 years ago, Moody et al10 studied 22 postmortem brains from older adults and showed that 65% had periventricular venous collagenosis of the small deep venules identified by trichrome staining; of these, 77% had severe WMH, as detected on antemortem scans. More recently, Keith et al12 extended these results, primarily implicating the large caliber (>200 µm in diameter) deep penetrating venules in a postmortem sample of 24 patients with dementia known to have periventricular white matter disease and 18 controls without dementia. While collagenosis of venules (small- and medium-caliber deep medullary venules) and myelin pallor (a marker of demyelination) also correlated with WMH volume, in a multivariate analysis, stenosis of the large-diameter venules best predicted WMH volumes. The same lab group also previously corroborated these results in vivo, using novel imaging techniques to visualize intraparenchymal venules or perivascular spaces, similarly describing an association between enlargement of these vessels and WMH volume.24
Yan et al25 used susceptibility-weighted MR imaging to quantify venulopathy of the deep medullary veins on the basis of voxel-intensity projection images. They found a relationship between WMH volume and large-caliber deep medullary vein visibility, which may be a manifestation of venous collagenosis. In a follow-up study, this same group created a visual grading score of deep medullary veins based on the venous continuity and homogeneity and found that scores were higher in subjects with higher WMH volume.26
Our study is the first to find an association between WMH and larger cerebral veins, namely the internal cerebral veins and the basal veins of Rosenthal. In the brain, the veins are valveless, and the draining system begins in the postcapillary venule and drains into venules, collecting veins, major parenchymal veins, the extraparenchymal veins, and finally the major dural sinuses. In particular, the periventricular veins drain into both the deep medullary veins and the internal cerebral veins and basal veins of Rosenthal. Moody et al10 hypothesized that periventricular venous collagenosis increases venous pressure, thus shunting blood away from these larger veins. Understanding the pathophysiology underlying the association between venous diameter and increased WMH, a traditional marker of small artery disease, may help us broaden the vascular contributions to AD and related dementias. We must interpret this association cautiously because we do not have histologic data to provide evidence as to what may be contributing to this increased diameter.
It is likely that venous collagenosis in aging is driven by chronic ischemia in the periventricular white matter from chronic occlusive arteriopathies, resulting from vascular risk factors such as hypertension and diabetes, but also possibly amyloid angiopathy, arteriolar tortuosity related to age, and other arterial pathologies.27,28 Arterial occlusive diseases exacerbate the gradient of diminishing cerebral blood flow toward the periventricular white matter, and this is especially evident in the watershed zones at the frontal and posterior horns of the ventricles.29 This chronic ischemia leads to venous collagenosis, which makes the deep medullary venules leaky and less able to absorb fluid. Collagenosis also increases venous resistance, which can further impair perfusion and interstitial flow and may also impede perivascular clearance of toxic proteins such as amyloid.12 The resulting venous hypertension could also increase pressure in the adjacent internal cerebral veins and basal veins, enlarging their diameters, thus causing them to become dilated in some individuals. This effect may be dampened in the vein of Galen because it is much larger and drains venous blood from a number of other regions, including the basal ganglia, thalamus, and occipital regions. The straight sinus and superior sagittal sinus are lined by the dura and would, therefore, not be expected to change in size with increased venous pressure.
Typically, cerebral venous dilatory anomalies are described as developmental in nature, usually resulting in severe complications that present neonatally or in early childhood. To our knowledge, the only descriptions of enlarged internal cerebral veins or basal veins of Rosenthal in the older population have been in cases of cerebral venous thrombosis, a clinically symptomatic condition that often presents with headache, stroke symptoms, and seizures.30⇓–32 Some type of pathology downstream of the veins, such as increased resistance, stenosis, or vessel tortuosity, could result in reflux and subsequent dilation, similar to what is seen in varicose veins. Vein of Galen malformations and aneurysmal dilations, for example, both congenital anomalies, result in dilation of the internal cerebral veins and basal veins of Rosenthal due to reflux.33
Another possibility is that a larger vein diameter may result from the dilated perivascular space around it. An enlarged perivascular space has been described as a feature of cerebral small-vessel disease, with specific association with lacunar ischemic stroke and WMHs.34 Likewise, a strong association has been observed between the severity of dilation of perivascular spaces and the severity of AD, and this relationship correlates to β amyloid load in the cortex.35 Most interesting, venous collagenosis in the deep white matter has been observed in CADASIL,28 a syndrome that also exhibits dilated perivascular spaces.36 Furthermore, perivascular spaces are thought to be involved in the clearing of interstitial solutes such as β amyloid via the glymphatic system.37 Thus, if venous dilation were a surrogate marker of dilated perivascular spaces, the associations that we found would have biologic plausibility as suggested by current literature. We are currently developing techniques for perivascular space quantification, which we hope to use in future studies.
The issue of multiple comparisons is a potential limitation to the study, but most of the observed effects were similar in magnitude and predicted direction; therefore, we do not believe that they were merely a result of a type I error. SWI is a noninvasive technique that allows vein visualization in vivo. The “blooming effect” is a phenomenon known to occur in SWI scans, in which certain hypointense signals become amplified. Although blooming may have played a role in the absolute values that we were able to measure, all SWI scans were acquired under identical, controlled parameters and any blooming impact should be consistent across all subjects.
Conclusions
Overall, this study provides evidence of the relationship between WMHs and both the internal cerebral veins and the basal veins of Rosenthal. It is unclear whether this association is merely correlative or if there is a causation between these pathologies. There are many avenues that can expand on this research in the future. As mentioned above, our lab is in the process of developing methods for perivascular space quantification, which could be used to determine whether these spaces relate to cerebral venous diameter. Longitudinal comparative analyses and/or comparisons across age groups could provide evidence of a temporal relationship. We could also examine specific CSF biomarkers of AD to see whether there is an association between vein/sinus diameter and CSF amyloid and τ. Our cohort consists of randomly recruited individuals without dementia diagnoses; an analysis of subjects with MCI and AD could provide a more nuanced understanding of this relationship. Other techniques such as angiography could depict what is happening internally within the vasculature to help delineate the pathology, though costs and physical risks could provide limitations. With the aging population, the clinical implications are clear: Understanding the etiology of this relationship might lead to therapeutic and preventative techniques to combat vascular disease and its downstream effects.
Acknowledgments
We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study.
Footnotes
Data collection and sharing for the project were supported by the Washington Heights–Inwood Columbia Aging Project (WHICAP, P01AG07232, R01AG037212, RF1AG054023, R01AG034189, R01AG054520, and R56AG034189) funded by the National Institute on Aging and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant No. UL1TR001873.
This article has been reviewed by Washington Heights–Inwood Columbia Aging Project investigators for scientific content and consistency of data interpretation with previous Washington Heights–Inwood Columbia Aging Project study publications.
Disclosures: Jose Gutierrez—RELATED: Grant: National Institute on Aging*; UNRELATED: Consultancy: specialist on call, law firm for medical litigation; Employment: Columbia University; Grants/Grants Pending: National Institute on Aging.* Sandra E. Black—UNRELATED: Consultancy: Novartis, Merck, Eli Lilly, Pfizer, Roche, Comments: ad hoc consultancy; Grants/Grants Pending: GE Healthcare, Eli Lilly, Biogen Idec, Novartis, Genentech, Optina, Roche*; Payment for Lectures Including Service on Speakers Bureaus: Medscape/Biogen, Eli Lilly, Novartis, Comments: Continuing Medical Education. *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 31, 2019.
- Accepted after revision August 1, 2019.
- © 2019 by American Journal of Neuroradiology