Abstract
BACKGROUND AND PURPOSE: Many studies have shown that insomnia is an independent factor in cognitive impairment, but the involved neurobiological mechanisms remain unclear. We used regional homogeneity to explore the specific neurobiologic indicators of chronic insomnia disorder with mild cognitive impairment.
MATERIALS AND METHODS: Thirty-nine patients with insomnia were divided into a group with and without cognitive impairment; we also included a control group (n = 28). Abnormalities in brain functional activity were identified by comparing the regional homogeneity values for each brain region among the groups.
RESULTS: Subjective insomnia scores were negatively correlated with cognitive impairment after controlling for age, sex, and educational effects. Regions with significant differences in regional homogeneity values in the 3 groups were concentrated in the right medial prefrontal cortex, the right superior frontal gyrus, and the left superior occipital gyrus. Meanwhile, subjective insomnia scores were negatively correlated with the strength of the decreased regional homogeneity in the right medial prefrontal cortex. The increased regional homogeneity value in the right superior frontal gyrus was positively correlated with the Montreal Cognitive Assessment score in patients.
CONCLUSIONS: Our results indicate that decreased regional homogeneity values in the medial prefrontal cortex and increased regional homogeneity values in the cuneus may be important neurobiologic indicators of chronic insomnia disorder and accompanying cognitive impairment. Overall, our study described the regional homogeneity of the whole brain in chronic insomnia disorder with mild cognitive impairment and could be the basis for future studies.
ABBREVIATIONS:
- BA
- Brodmann area
- CID
- chronic insomnia disorder
- MCI
- mild cognitive impairment
- mPFC
- medial prefrontal cortex
- NC
- healthy control
- NI
- no impairment
- ReHo
- regional homogeneity
- SFG
- superior frontal gyrus
The relationship between insomnia and cognitive function has attracted considerable attention in recent years. Large-sample meta-analyses have shown that patients with insomnia have mild or moderate dysfunction in attention, episodic memory, working memory, and executive function compared with healthy controls.1 A number of neuropsychological studies have found that older patients with chronic insomnia disorder (CID) have significant deficits in cognitive function compared with individuals of the same age without insomnia symptoms.1⇓⇓–4 Although some scholars have proposed that insomnia is associated with normal aging5 or neurodegenerative changes,6,7 recent research indicates that insomnia is an independent factor in cognitive impairment.8⇓⇓–11
Using [18F] FDG-PET, the earliest study found that the interacting neural networks of patients with insomnia were mainly distributed in the awakening, affective control, and cognitive systems.12 The observed abnormalities in the hippocampus and medial prefrontal cortex (mPFC) were consistent with the clinical features of cognitive impairment in patients with insomnia and the results of neurophysiology and neuroendocrine studies, indicating that memory integration is impaired in insomnia.13⇓–15 Considering similarities in neuromodulatory factors and their mechanisms of action sites in insomnia and Alzheimer disease, a possibility that has attracted much attention from neurologists, neuroscientists, and neuroradiologists is whether insomnia and Alzheimer disease share the same pathogenesis.16,17
fMRI provides a primary method of mechanism detection in insomnia. Some researchers have explored network mechanisms underlying decreased working memory and executive dysfunction in insomnia using task-state fMRI.18⇓–20 They have found decreased activity in the frontoparietal cortex18 and an abnormal frontal-striatal network during task-state in patients with insomnia.19 Furthermore, the activity of the medial prefrontal lobe could be recovered following insomnia improvement.20 Behavioral and fMRI studies have shown that the impairment of the executive control network in patients with CID is associated with reduced nocturnal slow-wave sleep time,21 which is consistent with the impairments in the prefrontal and thalamus attention networks during sleep deprivation.13,22,23 Moreover, PET and fMRI studies have yielded similar results.24,25 Although the above studies suggest that insomnia may be the potential reason for cognitive impairment, the involved mechanisms in patients with insomnia remain unclear.
Compared with task-state fMRI, resting-state fMRI can be used to disregard differences in brain activation caused by inconsistencies in task performance and may be used as a reflection of the real changes within inherent brain activity and/or the endogenous neurophysiologic process of the patients' brains under the awake state. The regional homogeneity (ReHo) method can effectively evaluate resting-state brain activity across the whole brain of an individual and has good reproducibility.26,27 The method has been widely used in the study of resting-state brain functional imaging for neurodegenerative diseases, emotional diseases, and cognitive function.
Several previous studies have investigated the regional spontaneous activity patterns in patients with insomnia. These studies have found that patients with insomnia have abnormal spontaneous activity in specific regions, including the insula, cingulate gyrus, fusiform gyrus, and cerebellum.28,29 In addition, these altered ReHo values are associated with sleep quality and psychological scores30; these findings suggest that the abnormal ReHo values of specific regions could reflect the brain mechanism of emotional disorders in patients with insomnia. Moreover, neuroimaging studies have shown that abnormal brain regional homogeneity is an important marker of cognitive impairment in patients with Alzheimer disease31,32 and could accurately reflect the severity of cognitive impairment.33
We diagnosed mild cognitive impairment (MCI) according to the Peterson MCI standard, and patients with insomnia were divided into the cognitive impairment (CID-MCI) group or the group without cognitive impairment (CID-NI). Then, we used the ReHo method to explore differences in regional spontaneous activity in the whole brain between the healthy control (NC), CID-NI, and CID-MCI groups. We hypothesized that the ReHo index would differ among the NC, CID-NI, and CID-MCI groups and that the differences in ReHo would be associated with differences in cognitive ability. A post hoc analysis was then performed to compare the ReHo index between each pair of groups. Finally, a correlation analysis was performed between the ReHo index of the identified regions and various clinical variables in the CID-NI and CID-MCI groups to evaluate the relationship between the ReHo scores and the cognitive abilities of the CID-NI and CID-MCI groups.
Materials and Methods
The participants in the present study also composed the sample in a previous study of spontaneous activity measured by whole-brain functional connectivity.34 All subjects met identical methodologic stringency criteria; comprehensive clinical details can be found in the prior work.34
Participants
Patients with insomnia and volunteers were enrolled from a neurology clinic. The participants underwent a series of examinations, including a clinical interview, laboratory blood tests, and neuropsychological assessment. Consent forms were signed by the participants before the study, and the study protocol was approved by the ethics committee.
All participants underwent a complete physical and neurologic examination, standard laboratory tests, and an extensive battery of neuropsychological assessments, which included the Pittsburgh Sleep Quality Index, Insomnia Severity Index, Hamilton Anxiety Scale, Hamilton Depression Rating Scale, Mini-Mental State Examination, Montreal Cognitive Assessment, and Clinical Dementia Rating. Patients with CID also underwent polysomnography.
The diagnosis of CID met the criteria of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders and the third edition of the International Classification of Sleep Disorders.35 Although chronic insomnia is not exactly equal to primary insomnia, we did not find severe anxiety and depression disorders in the included patients.
The On-line Table presents the demographic, neuropsychological, and sleep characteristics of the enrolled participants.
Study Method
The patients with CID completed subjective and objective sleep-quality assessments (Pittsburgh Sleep Quality Index and Insomnia Severity Index scales and Polysomnography monitoring). Sleep time data were analyzed and calculated by an experienced technician and were reviewed by a neurologist. These data included total sleep time, sleep-onset latency, wake time, nonrapid eye movement slow-wave activity (S3 + S4) time and latency, and rapid eye movement sleep time and latency. The details of the neuropsychological assessments are provided in the On-line Table.
MR Imaging Acquisition
Briefly, MR imaging was performed using a 1.5T superconductor MR imaging scanner (Intera Achieva; Philips Healthcare, Best, the Netherlands). The parameters and scanning mode of the MR imaging in this study can be found in the previously published study.34
MR Imaging Data Preprocessing
The fMRI data were preprocessed with a method consistent with protocols in previously published studies using the BRAinNetome fMRI Toolkit (Brant; http://brant.brainnetome.org). The preprocessing steps included the following: 1) slice-timing, 2) realignment to reduce head motion, 3) normalization to a standard EPI template and reslicing to 2 × 2 × 2 mm cubic voxels, 4) denoising by regressing out several effects (6 motion parameters, linear drift, and the mean time-series of all voxels within the white matter and CSF), and 5) temporal filtering (0.01–0.08 Hz) to reduce noise.
Estimation of Interregional Functional Connectivity–ReHo Index
ReHo provides a fast mapping of the regional activity across the whole brain.26 For each subject, the ReHo map was normalized by dividing it by the mean ReHo of the whole brain for each subject to reduce the effect of individual variability,36,37 for each voxel: ReHonormlized = ReHo (x, y, z) / Mean (ReHo).
Statistical Analysis
A 1-way ANOVA with age and sex as covariances was performed to identify the differences among the CID-MCI, CID-NI, and NC groups. The resultant F value map was then thresholded using P < .001 (F = 7.76, two df, 60 df for each voxel and a cluster size of at least 60 voxels, uncorrected). Subsequently, the regions that showed significant differences were extracted as ROIs, and the mean ReHo values were used for a post hoc analysis. Statistical comparisons of the mean ReHo values between each pair of groups were performed using a 2-sample 2-tailed t test at a threshold of P < .05.
To determine whether the ReHo index varied with disease progression in the CID-MCI and CID-NI groups, we performed correlation analyses between the ReHo index and each of the clinical variables (Mini-Mental State Examination, Pittsburgh Sleep Quality Index, and Hamilton Anxiety Scale scores). Because these analyses were exploratory in nature, we used a statistical significance level of P < .05 (uncorrected).
Results
Group Differences
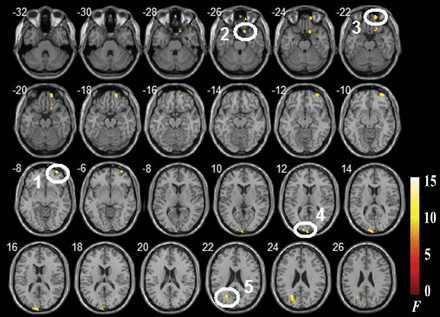
A 1-way ANOVA was used to determine the regions in which the ReHo index was significantly altered among the CID-NI, CID-MCI, and NC groups. We found that the ReHo index was significantly different in the following regions: the right mPFC (inferior frontal gyrus, orbital middle frontal gyrus, and Brodmann areas [BAs] 47 and 11), the right superior frontal gyrus (SFG; BA 11), the left cuneus (BA 18), and the left superior occipital gyrus (BAs 31 and 18) among the CID-NI, CID-MCI, and NC groups (Table and Fig 1).
Regions with differences in ReHo in the CID-NI, CID-MCI, and NC groups, and their coordinatesa
Regions with differences in regional homogeneity among the CID-NI, CID-MCI, and NC groups (voxels at least 60, P < .001). 1) Right inferior frontal gyrus. 2) Right superior frontal gyrus. 3) Right orbital middle frontal gyrus. 4) Left cuneus. 5) Left superior occipital gyrus.
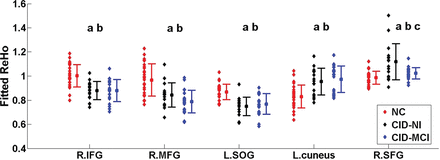
As Fig 2 shows, the mean ReHo values in the mPFC decreased significantly (P < .05) in the CID-NI and CID-MCI groups compared with the NC group, while the mean ReHo values in the cuneus increased significantly (P < .05) in the CID-NI and CID-MCI groups compared with the NC group. In addition, the mean ReHo value in the right SFG significantly increased (P < .05) in the CID-NI group compared with the CID-MCI and the NC groups.
Plot of the regional homogeneity index among the CID-NI, CID-MCI, and NC groups in the identified brain regions (voxels at least 60, P < .001). a, The ReHo index is significantly different between the NC and CID-MCI groups. b, The ReHo index is significantly different between the NC and CID-NI groups. c, The ReHo index is significantly different between the CID-MCI and CID-NI groups. R indicates right; L, left; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; SOG, superior occipital gyrus.
Relationship between ReHo and Clinical Variables
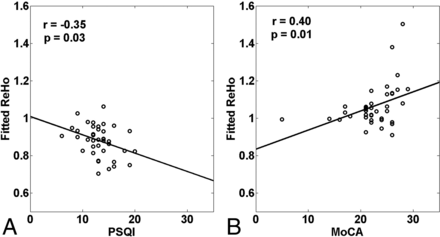
As Fig 3 shows, the strength of the ReHo score was negatively correlated with the Pittsburgh Sleep Quality Index ratings (r = −0.35, P = .03) in the right inferior frontal gyrus in patients with CID. The strength of the ReHo index was positively correlated with the Montreal Cognitive Assessment ratings in the right superior frontal gyrus (r = 0.40, P = .01).
Correlations between altered regional homogeneity patterns and subjective sleep scores and cognitive scores in the chronic insomnia disorder group (P < .05). A, Right inferior frontal gyrus. B, Right SFG. Although the 2 points in the figure (B) look like outliers, the high correlation was also obtained without them (r = 0.36, P = .03). PSQI indicates the Pittsburgh Sleep Quality Index; MoCA, the Montreal Cognitive Assessment.
Discussion
To the best of our knowledge, this is the first study to investigate the ReHo index of brain spontaneous activity in patients with both CID-MCI and CID-NI as well as to compare them with NCs. Significant differences were found in the ReHo scores in various brain regions—that is, the right mPFC (inferior frontal gyrus, orbital middle frontal gyrus), the right SFG, the left cuneus, and the left superior occipital gyrus among the CID-MCI, CID-NI, and NC groups (Table and Fig 1). Furthermore, the ReHo index in these identified brain regions showed a significant correlation with clinical variables in the CID groups (Fig 3).
We observed that regions with decreased ReHo were located in the right mPFC (inferior frontal gyrus, orbital middle frontal gyrus) in the CID-MCI and CID-NI groups compared with the NC group. Many studies have demonstrated that the mPFC plays a pivotal role in mediating sleep and generating nonrapid eye movement slow-wave oscillations.38⇓⇓–41 Recent electroencephalography and fMRI studies have shown that the waking metabolism rate and reduced gray matter volumes in the medial frontal gyrus of humans are both related to aging and closely related to nighttime slow-sleep intensity.38,42 Previous morphometry studies have found that patients with chronic insomnia displayed significantly reduced gray matter volumes in the orbitofrontal cortex (BAs 10 and 11) and medial frontal lobe,43,44 and that the gray matter volumes in the orbitofrontal cortex are positively correlated with the severity of insomnia in patients with chronic insomnia.44 Furthermore, an fMRI study found that the activity of the medial prefrontal lobe could be recovered after the insomnia improved.20 All these results strengthen the evidence for insomnia-related changes in the mPFC in this study. Moreover, this correlation was supported by the negative relationship between the ReHo index values in the right inferior frontal gyrus and scores on the Pittsburgh Sleep Quality Index (Fig 3).
Considering that the mPFC is a key region in the default mode network that characterizes autobiographic memory retrieval,45 our results further suggest that ReHo values in the mPFC can reflect disrupted global cognitive function in patients with CID-MCI. Consistent with our findings, several previous studies have found decreased connectivity in the prefrontal cortex,22 internal default network, and between the default network and its negative feedback network after short-term sleep deprivation in healthy individuals.46 In recent years, impaired connectivity in the default mode network has been found to be common in patients with insomnia.34,46⇓–48 Moreover, slow-wave sleep plays an important role in memory integration and storage.13⇓–15,41,49,50 Some studies have reported that structural and functional destruction in the mPFC, which is known as the major region generating slow-wave sleep oscillations, could destroy the memory systems.11,38,39 In this study, the ReHo values in the right orbital middle frontal gyrus were lower in the CID-MCI than in the CID-NI group (Fig 2). This finding indicates that the coherence in the regional activity of the mPFC gives an expression of affected memory systems induced by CID. Moreover, the patients in the CID-MCI group had lower nonrapid eye movement slow-wave activity (S3 + S4) (%) and were older than the patients in the CID-NI group (On-line Table). Combined with previous experimental results, our observations suggest that the disruption of spontaneous brain activity in the mPFC due to insomnia may be accelerated with aging, or shortened nonrapid eye movement slow-wave activity (slow wave activity) and aging may synergistically disrupt certain cognitive abilities.11,39 Taken together, the decreased homogeneity in the mPFC may be a characteristic alteration in the patients with CID-MCI.
We also found an increased ReHo in the left cuneus in the CID-NI and the CID-MCI groups compared with the NC group (Fig 2). Several neuroimaging studies have found abnormal metabolism and dysfunction in the occipital lobe in patients with insomnia.51,52 Although these results are inconsistent, both studies reported a negative correlation between γ aminobutyric acid content in the occipital lobe and sleep-onset latency, which suggests that the occipital lobe plays an important role in sleep-awakening mediation. In addition, previous studies have shown that patients with insomnia displayed significantly increased ReHo in the left cuneus compared with NCs.29 This conclusion is consistent with the results of our study, wherein we found that the ReHo of the left cuneus was increased in all patients with CID.
In the present study, the right SFG was the only region that could be used to distinguish the 3 groups. We found that the right SFG exhibited a significantly increased ReHo in both the CID-MCI and CID-NI groups compared with the NC group (Fig 2) and the ReHo index values in the SFG positively correlated with Montreal Cognitive Assessment ratings. In a recent study, patients with CID showed an increased positive correlation between the left SFG and ipsilateral parahippocampal gyrus, and the connectivity strength was positively correlated with the Mini-Mental State Examination scores.34 This result suggests that increased functional connectivity of the SFG could compensate for the cognitive impairment after prefrontal disconnection. In addition, 2 studies using the ReHo method have observed that patients with insomnia showed altered spontaneous activity in extensive emotional brain regions (including the insula, cingulate gyrus, fusiform gyrus, temporal lobe, cerebellum, and frontal lobe).28,29 Wang et al29 found that altered ReHo values (the left insula, the right middle cingulated cortex, and the right precentral gyrus) are associated with psychological scores, while Dai et al28 considered the decreased ReHo values in the SFG to be a marker for cognitive and emotional dysfunction in insomnia. Moreover, the patients in the CID-NI group had both the highest ReHo values in the SFG and the most severe clinical manifestations of difficulty with sleep onset and abnormal emotions compared with the CID-MCI and NC groups; this finding is consistent with the cortical hyperarousal and emotional disorders hypothesis.53 Taken together, our results further indicate that ReHo values in the right SFG can reflect the degree of difficulty with sleep onset or the hyperarousal state in patients with CID.
Some limitations should be borne in mind when interpreting the results. No regions showed significantly decreased ReHo scores in the CID-MCI group compared with the CID-NI group, while the right orbital middle frontal gyrus had a significantly more destructive tendency in the CID-MCI than in the CID-NI and NC groups (Fig 2). In addition, more data from sleep-monitoring indicators, such as nonrapid eye movement slow-wave activity and rapid eye movement sleep duration, sleep latency, and band characteristics, as well as analysis of correlations between electrophysiology and fMRI measures and cognitive ability, are required for further studies. The present study showed the brain functional changes and clinical indices of CID-MCI and CID-NI in the 2 insomnia subgroups, but not in patients with pure MCI. Further studies could consider including patients with amnesic MCI to better explain the sleep and cognitive decline effect and may help us understand the pathogenetic process that leads from insomnia to Alzheimer disease or of the aggravation of insomnia, which could explain the phenomenon of patients with insomnia having an increased incidence rate of Alzheimer disease compared with individuals without insomnia.
Conclusions
This study is the first to examine the spontaneous brain activity of patients with CID-MCI, to our knowledge. Our results indicate that the decreased ReHo values observed in the mPFC of patients may be an important neurobiologic indicator of CID and accompanying cognitive impairment and that the enhanced local homogeneity observed in the right SFG may act as a predictor of both destruction in emotional moderation and the degree of hyperarousal state. Overall, our study describes the regional homogeneity of the whole brain in patients with CID-MCI and provides a foundation for future related studies.
Ethical Approval and Informed Consent
This experiment was conducted on humans.
Approval: All experimental protocols were approved by the Clinical Research Ethics Committee of Dongfang Hospital of Beijing University of Chinese Medicine.
Accordance: The methods were carried out in accordance with the approved guidelines.
Informed consent: Informed consent was obtained from all participants before participation.
Acknowledgments
We thank all authors of the included studies. We especially thank Dr Yunling Zhang for his kind help and suggestions. Moreover, we would like to thank Editage (www.editage.com) for English language editing.
Footnotes
This work was supported by a grant from Beijing University of Chinese Medicine (2017-JYB-JS-166) and grants from the National Natural Science Foundation of China (81471649, 81571648 and 81370037).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received September 15, 2017.
- Accepted after revision December 29, 2017.
- © 2018 by American Journal of Neuroradiology