Abstract
BACKGROUND AND PURPOSE: Endovascular treatment has emerged as a minimally invasive technique for patients with acute ischemic stroke to achieve recanalization. Our aim was to determine the effects of endovascular treatment on clinical and safety outcomes compared with best medical treatment.
MATERIALS AND METHODS: Fifteen randomized trials that compared endovascular treatment with best medical treatment in patients with acute ischemic stroke met the inclusion criteria. We calculated pooled odds ratios and 95% CIs by using random-effects models. The primary end point was a favorable outcome defined by a modified Rankin Scale score of 0 (no symptoms), 1 (no significant disability), or 2 (slight disability) at 90 days postrandomization.
RESULTS: Of the 2980 subjects randomized, the proportion of subjects who achieved a favorable outcome was significantly greater among those randomized to endovascular treatment compared with best medical treatment (2949 subjects analyzed; odds ratio, 1.82; 95% CI, 1.38–2.40; P < .001). Excellent outcome (modified Rankin Scale score of 0 or 1) was also significantly greater among those randomized to endovascular treatment (2791 subjects analyzed; odds ratio, 1.77; 95% CI, 1.29–2.43, P < .001). Risk of symptomatic intracranial hemorrhage was similar between endovascular treatment and best medical treatment (2906 subjects analyzed; odds ratio, 1.19; 95% CI, 0.84–1.68; P = .34).
CONCLUSIONS: Compared with best medical treatment, the odds of achieving a favorable outcome or excellent outcome at 3 months postrandomization are approximately 80% higher with endovascular treatment among patients with acute ischemic stroke.
ABBREVIATION:
- ICH
- intracranial hemorrhage
Endovascular treatment was introduced for patients with ischemic stroke in whom limited benefit with intravenous recombinant tissue plasminogen activator was expected or for those in whom IV thrombolytics was not indicated. There has been a 6-fold increase in the use of endovascular treatment among patients with acute ischemic stroke in the past few years,1 and availability of endovascular treatment has been identified as a mandatory component of comprehensive stroke centers in the United States.2,3 Several randomized trials have compared the efficacy of endovascular treatment with best medical treatment, which may include IV thrombolytic administration. Because of the small sample sizes or the limited representation of patients most likely to benefit from endovascular treatment within a study population, the results have been conflicting.4⇓⇓–7 We performed this meta-analysis to combine the results of all existing trials to provide a comprehensive assessment of the benefit and risk associated with endovascular treatment in patients with acute ischemic stroke.
Materials and Methods
Study Design
We performed a meta-analysis of relevant randomized controlled trials and stratified analyses by important differences in trial characteristics. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We performed a computerized literature search of the Medline and Cochrane data bases on April 17, 2015, with the following search terms: “endovascular treatment,” “thrombectomy devices,” “acute ischemic stroke,” “proximal intracranial occlusion in the anterior circulation,” “randomized control trial,” “intra-arterial revascularization,” “retrievable stents,” “alteplase,” “endovascular thrombectomy with the Solitaire FR stent retriever,” “recombinant pro-urokinase,” and “intravenous and intra-arterial recombinant tissue plasminogen activator (rtPA).” No other search restrictions were applied.
We included trials if they enrolled patients with acute ischemic stroke (within 24 hours of symptom onset) for endovascular treatment (intra-arterial thrombolysis and mechanical thrombectomy alone or in combination) and randomly assigned patients to endovascular treatment or medical treatment with or without IV thrombolysis. Trials in which endovascular treatment was performed after administering IV thrombolysis were included. Trials that included <10 subjects, those that did not report clinical outcomes according to grades of modified Rankin Scale postrandomization, or those that performed any procedure for prevention of new or recurrent ischemic stroke were excluded.
Outcomes
The primary efficacy end point was the proportion of randomized subjects who achieved a modified Rankin Scale score of 0 (no symptoms), 1 (no significant disability), or 2 (slight disability) at 90 days postrandomization.8 Secondary efficacy end points were the proportion of randomized subjects who achieved a modified Rankin Scale score of 0 or 1 and survival at 3 months postrandomization. Posttreatment symptomatic intracranial hemorrhage was the safety end point analyzed. Information on these end points was abstracted by M.F.I. and H.A.R. independently and entered into a structured dataset and compared. All disagreements were resolved by reaching a consensus, and there was complete agreement on abstracted results in the final dataset.
Statistical Analysis
We calculated odds ratios and 95% CIs by using Comprehensive Meta-Analysis 2·2·048 (Biostat, Englewood, New Jersey) for each of the trials. We compared the calculated odds ratios with the odds ratios or hazard ratios reported in the original article when available to ensure congruence. If specific end points were not reported in a trial, that trial was excluded only from the pooled analyses of the specific end points that were not reported. We calculated pooled odds ratios by using a random-effects model by using the method of DerSimonian and Laird.9 Heterogeneity was assessed by using the Cochran Q statistic, and when there was heterogeneity, we assessed the magnitude of heterogeneity with the I2 measure (the percentage of total variability due to true between-study heterogeneity). We stratified results by key trial characteristics, including the type of subjects recruited (exclusively within 4.5 hours of symptom onset and/or confirmation of arterial occlusion before randomization), the type of endovascular treatment performed (intra-arterial thrombolysis or a combination of intra-arterial thrombolysis and mechanical thrombectomy or mechanical thrombectomy alone), the administration of IV thrombolysis (before endovascular treatment), and the treatment in subjects randomized to medical treatment (received IV thrombolysis).
In sensitivity analyses, we restricted the analyses to trials with at least 50 randomized subjects who achieved a modified Rankin Scale score of 0, 1, or 2 at 3 months postrandomization, and we analyzed for heterogeneity on the basis of masking within the trial. We analyzed the results only for trials that assessed the primary outcome at 90 days postrandomization by using blinded ascertainment. We assessed publication bias by visual inspection of funnel plots and by calculation of the P value (2-sided) for the Egger intercept. We did not make corrections for multiple hypotheses testing because of the exploratory nature of the analyses. All tests were 2-sided, with P < .05 deemed as significant.10
Results
We identified 18 randomized clinical trials evaluating endovascular treatment in patients with acute ischemic stroke (On-line Fig 1).11⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–28 Three trials26⇓–28 (87 subjects randomized) were excluded because they either used the Scandinavian Stroke Scale or the National Institutes of Health Stroke Scale as outcome measures or endovascular treatment was used in both treatment groups. The remaining 15 trials11⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–25 met the inclusion criteria and were included in the meta-analysis (see On-line Table 1), resulting in 2980 patients. One trial22 ascertained outcome at 6 months postrandomization, and we accepted the reported outcome as a surrogate for outcome at 90 days postrandomization. The characteristics of included studies are provided in the On-line Table. Six trials enrolled patients exclusively within ≤4.5 hours of symptom onset, and 12 trials required confirmed arterial occlusion before randomization (by conventional angiography in 3 and CT or MR angiography in 9). Endovascular treatment consisted of intra-arterial thrombolysis alone in 5 trials, and a combination of intra-arterial thrombolysis with mechanical thrombectomy or mechanical thrombectomy alone was used in 10 trials. Seven trials permitted the administration of IV thrombolysis before endovascular treatment. In 11 trials, subjects randomized to medical treatment received IV thrombolysis when indicated.
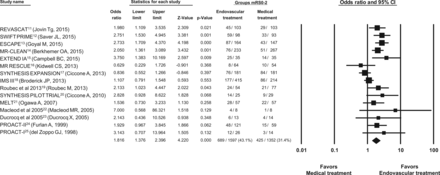
Among 2980 subjects randomized, 1114 (37.4%) achieved a modified Rankin Scale score of 0, 1, or 2 at 3 months postrandomization. The proportion of randomized subjects with acute ischemic stroke who achieved a modified Rankin Scale score of 0, 1, or 2 at 90 days postrandomization was significantly greater among those randomized to endovascular treatment (689 [43.1%] of 1597 subjects) compared with best medical treatment (425 [31.4%] of 1352 subjects) (2949 subjects analyzed; odds ratio, 1.82; 95% CI, 1.38–2.40; P < .001) as demonstrated in Fig 1. However, there was significant heterogeneity among the trials (Cochran Q statistic, 34.35; 14 df; P = .002; I2 = 59.24%). In the first sensitivity analysis, the proportion of randomized subjects with acute ischemic stroke who achieved a modified Rankin Scale score of 0, 1, or 2 at 3 months postrandomization was significantly greater among those randomized to endovascular treatment (2906 subjects analyzed; odds ratio, 1.78; 95% CI, 1.34–2.37; P < .001) after exclusion of trials that had <50 subjects who achieved a modified Rankin Scale score of 0, 1, or 2 at 3 months postrandomization. In the second sensitivity analysis, the results were unchanged after exclusion of trials that did not use blinded outcome ascertainment or did not assess outcome at 90 days postrandomization (2818 subjects analyzed; odds ratio, 1.80; 95% CI, 1.34–2.42; P < .001).
Odds of favorable outcome (modified Rankin Scale scores, 0, 1, or 2) at 90 days postrandomization.
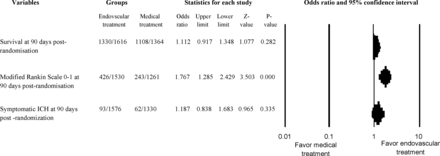
The proportion of randomized subjects with acute ischemic stroke who achieved a modified Rankin Scale score of 0 or 1 at 90 days postrandomization was significantly greater among those randomized to endovascular treatment (426 [27.8%] of 1530 subjects) compared with best medical treatment (243 [19.3%] of 1261 subjects) (2791 subjects analyzed; odds ratio, 1.77; 95% CI, 1.29–2.43; P < .001; Fig 2 and On-line Fig 2). There was significant heterogeneity among the trials (Cochran Q statistic, 27.79; 12 df; P = .006; I2 = 56.83%). There was no difference in survival at 90 days postrandomization between subjects randomized to endovascular treatment compared with those randomized to best medical treatment (2980 subjects analyzed; odds ratio, 1.11; 95% CI, 0.92–1.35; P = .28; Fig 2). The specific definition of symptomatic intracranial hemorrhage varied among trials (On-line Table 2), but 155 patients had a symptomatic intracranial hemorrhage. In pooled analyses, there was no difference in the risk of symptomatic intracranial hemorrhage between subjects randomized to endovascular treatment and those randomized to medical treatment (2906 subjects analyzed; odds ratio, 1.19; 95% CI, 0.84–1.68; P = .34). There was no heterogeneity among the trials concerning survival (Cochran Q statistic, 10.98;14 df; P = .687; I2 = 0%) or symptomatic intracerebral hemorrhage (Cochran Q statistic, 8.52; 12 df; P = .744; I2 = 0%).
Odds of excellent outcome (modified Rankin Scale scores, 0 or 1), survival at 90 days postrandomization, and posttreatment intracranial hemorrhage.
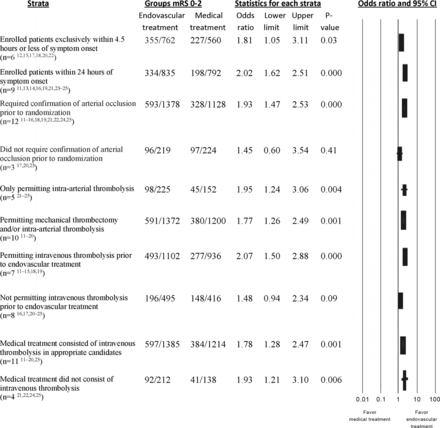
The odds ratios in various strata based on trial design, defined by type of subjects recruited, endovascular treatment performed, and treatment in subjects randomized to medical treatment, are provided in Fig 3. The odds of favorable outcomes were somewhat higher in trials that enrolled subjects within 24 hours of symptom onset (1627 subjects analyzed; OR, 2.02; 95% CI, 1.62–2.51; P < .001) compared with those that exclusively recruited within 4.5 hours of symptom onset (1322 subjects analyzed; OR, 1.81; 95% CI, 1.05–3.11; P = .03), with nonsignificant test for heterogeneity (Cochran Q statistic, 7.635; 8 df; P = .470; I2 = 0.000%). Notably, the patient populations are not independent because trials that included subjects within 24 hours also included those enrolled within 4.5 hours. The odds of favorable outcome were somewhat higher in 12 trials that required angiographic confirmation of arterial occlusion before randomization (2506 subjects analyzed; OR, 1.93; 95% CI, 1.47–2.53; P < .001) but not in the 3 trials that did not require confirmation of arterial occlusion (443 subjects analyzed; OR, 1.45; 95% CI, 0.60–3.54; P = .41). The odds of favorable outcome were higher in trials that permitted a combination of pharmacologic thrombolysis with mechanical thrombectomy or mechanical thrombectomy alone (2572 subjects analyzed; OR, 1.77; 95% CI, 1.26–2.49; P = .001) and those that permitted intra-arterial thrombolytic treatment alone (377 subjects analyzed; OR, 1.95; 95% CI, 1.24–3.06; P = .004). The odds of favorable outcome appeared higher with endovascular treatment in trials that permitted IV thrombolytic treatment before or with endovascular treatment than in trials that did not permit IV thrombolysis before endovascular treatment.
Odds of favorable outcome (modified Rankin Scale scores, 0, 1, or 2) at 90 days postrandomization in various strata based on trial design.
There was a trend toward higher odds of symptomatic intracranial hemorrhage in trials that enrolled subjects within 24 hours of symptom onset (1600 subjects analyzed; OR, 1.48; 95% CI, 0.91–2.40; P = .11; On-line Table 3) but not in those trials that exclusively recruited within 4.5 hours of symptom onset. The odds of symptomatic intracranial hemorrhage were higher in trials that permitted only intra-arterial thrombolytic treatment (377 subjects analyzed; OR, 4.19; 95% CI, 1.42–12.31; P = .009) but not in trials that permitted a combination of pharmacologic thrombolysis and mechanical thrombectomy or mechanical thrombectomy alone. There was no difference in 90-day survival with endovascular treatment in any of the strata based on trial design (On-line Table 3).
There was no evidence of publication bias having a significant effect on the results (Egger regression intercept P value [2-tailed] = 0.13; On-line Fig 3).
Discussion
We demonstrate the therapeutic benefit of endovascular treatment in 2980 subjects with acute ischemic stroke randomized in 15 controlled trials. The analysis included data from more recent trials in contrast to previous meta-analyses and systematic reviews.29,30 Such a design allowed incorporation of technologic advancements and larger sample sizes within the analysis. The magnitude of benefit associated with endovascular treatment appeared higher in more recent trials (Fig 1), presumably due to the use of new thrombectomy devices such as stent retrievers and appropriate patient selection. The odds of a favorable outcome were higher in trials that required angiographic confirmation of arterial occlusion before randomization. In a subset analysis of the Interventional Management of Stroke III trial,31 when only those subjects with arterial occlusion before randomization were analyzed, the magnitude of benefit with endovascular treatment was higher among such subjects (7% absolute increase in favorable outcome, P = .011 by ordinal shift analysis). The odds of a favorable outcome were higher with endovascular treatment, even in trials that enrolled subjects after 4.5 hours of symptom onset and those trials that enrolled subjects after receiving IV thrombolytics.
Some issues should be considered before interpretation of the results of the meta-analysis. We observed significant heterogeneity among results as observed in previous systematic review or meta-analysis of other clinical trials because of either clinical or methodologic diversity.32 Because of significant heterogeneity among studies, we used a random-effects model to take into account both within- and between-study variability.33,34 The model assumes that the effect is not the same in all studies and provides a much wider confidence interval (compared with a fixed-effects model).33 We also attempted to provide explanatory data by performing stratified analyses by key trial characteristics and sensitivity analyses. We acknowledge that another option would be to just perform a narrative review, but we chose to perform a meta-analysis because these studies represented treatments in which the value of the average effect will be of interest.35,36 We used trial-level data because patient-level data were not available. Patient-level data are unlikely to change the overall findings but may provide insight into confounding effects of patient and procedure-related variables.
The possibility of publication bias cannot be completely excluded due to the borderline value for nonsignificance (Egger test, P = .13). There is a small chance that the estimate of the beneficial effect of endovascular treatment in patients with acute ischemic stroke may be exaggerated due to selective publication of trials with positive findings.37 We presented data for outcomes at 90 days because data were available at that time point in most trials and the time point has been used consistently in most trials of acute ischemic stroke.8 The analysis does not provide any data on the effect of endovascular treatment on quality of life, cognitive deficits, and 1-year death and disability. There are also trials that are either ongoing or whose results have not yet been published after peer review, such as Assess the Penumbra System in the Treatment of Acute Stroke (THERAPY), Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE), and Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke (THRACE), which were not included to avoid flaws such as failure to assess the methodologic quality of the included primary studies in this meta-analysis.38
Conclusions
Our results support the recent focused update in the American Heart Association/American Stroke Association guidelines39 strongly recommending that patients with acute ischemic stroke receive endovascular therapy with a stent retriever if they meet specified criteria (Class I; Level of Evidence A) .Our results also support administering IV thrombolysis in appropriate candidates and confirmation of major arterial occlusion before selection for endovascular treatment. The implementations of the results of the meta-analysis into clinical practice may vary in different settings on the basis of the availability of triage patterns, advanced imaging, and endovascular treatment.
Footnotes
Disclosures: Abraham P. Thomas—UNRELATED: Payment for Lectures (including service on Speakers Bureaus): Genentech; Other: TTI Home Health Care,* Accel at Herman Park,* Comments: Medical Directorship. *Money paid to the institution.
Adnan I. Qureshi was responsible for the literature search, study design, data analysis, data interpretation, figures, and manuscript writing and revisions. Muhammad F. Ishfaq was responsible for the literature search, figures, data collection, data analysis, data interpretation, manuscript revision, and study design. Haseeb A. Rahman conducted the literature search, data collection, data interpretation, and manuscript revision. Abraham P. Thomas conducted the literature search, data collection, data interpretation, and manuscript revision.
Abstract previously presented at: European Stroke Conference, May 12–15, 2015; Vienna, Austria.
References
- Received August 5, 2015.
- Accepted after revision December 1, 2015.
- © 2016 by American Journal of Neuroradiology