Abstract
BACKGROUND AND PURPOSE: Cognitive impairment is a common, disabling symptom of MS. We investigated the impact of cerebral perfusion and brain and lesion volumetry on cognitive performance in 45 patients with SPMS by using MR imaging.
MATERIALS AND METHODS: Cognition was assessed by using a standard battery, the Minimal Assessment of Cognitive Function in Multiple Sclerosis. qCBF and qCBV maps were analyzed by using SPM and PLS. SPM was also used to conduct the GM, WM, and WML volumetric analyses.
RESULTS: Both SPM and PLS demonstrated significantly reduced qCBV in the superior medial frontal cortex of impaired patients. PLS also revealed significantly lower qCBV in the bilateral thalami and caudate nuclei of impaired patients and identified a pattern of significantly attenuated qCBF similar to that of qCBV. Performance on the Symbol Digit Modalities Test, which assesses information-processing speed, correlated most strongly overall with cerebral perfusion. Focal (ie, voxelwise) analyses of GM, WM, and WML volume revealed no significant differences between patients with and without cognitive impairment, though global GM volume was significantly decreased and global WML volume was significantly increased in impaired patients.
CONCLUSIONS: These results suggest that cognitively impaired patients with SPMS exhibit robust perfusion deficits in cortical and subcortical GM and impaired processing speed.
ABBREVIATIONS:
- BA
- Brodmann area
- DSC
- dynamic susceptibility contrast
- FWE
- family-wise error
- GM
- gray matter
- kE
- extent threshold
- LV
- latent variable
- MNI
- Montreal Neurological Institute
- PLS
- Partial Least Squares
- qCBF
- quantitative CBF
- qCBV
- quantitative CBV
- SPM
- Statistical Parametric Mapping
- SPMS
- secondary–progressive MS
- WML
- white matter lesion
Cognitive impairment is a common feature of MS and occurs in an estimated 43%–65% of patients.1 The highest frequency of impairment has been observed in patients with SPMS.2 Cognitive dysfunction in patients with MS has been reported to disrupt employment and social functioning.3,4 Deficits are apparent in multiple cognitive domains, such as information-processing speed, episodic memory, working memory, and executive function, with impaired processing speed being the most frequently cited deficit in systematic reviews.5⇓⇓–8
MR imaging is the most frequently used neuroimaging technique in studies of cognitive impairment in patients with MS.5⇓⇓–8 MS has been traditionally viewed as a disease predominantly affecting WM, though only modest associations between T2-weighted hyperintense WML load and cognitive test performance have been reported.9 There is, however, mounting evidence of GM pathology in MS. Brain volume measures, particularly those relating to third ventricle width10 and thalamic volume,11 correlate better with neuropsychological test performance than WML outcomes. Other studies assessing cortical12 and prefrontal atrophy13 further support the argument that decreased GM volume is associated with impaired cognitive functioning in patients with MS. In addition, cortical/subcortical lesion load was greater in cognitively impaired patients with MS than in their nonimpaired counterparts.14 With double inversion recovery MR imaging, an increased number of cortical lesions were associated with impaired processing speed and visuospatial memory.15 Cortical lesion volume also predicted cognitive status independent of GM volume.16
By virtue of its high vascularity and metabolic activity, GM is inherently sensitive to perfusion perturbations induced by pathologic change. Perfusion imaging offers the opportunity to investigate the functional impact of GM pathology. Cerebral perfusion can be investigated by using various MR imaging techniques, such as fMRI, arterial spin-labeling, and DSC. While the endogenous contrast agents used by fMRI and arterial spin-labeling are less invasive, DSC imaging is more frequently performed in the clinical setting. The bookend technique is a novel DSC calibration methodology that more accurately quantifies cerebral perfusion by using pre- and postgadolinium “bookend” scans.17⇓–19
Only 2 prior cerebral perfusion studies have explicitly investigated cognitive impairment in MS.20,21 Using a quantitative DSC technique, the first study reported significant correlations between subcortical GM CBF and the Rey Complex Figure Test score and subcortical GM CBV and the Delis-Kaplan Executive Function System score, though cortical tissue was not assessed.20 The second study interrogated both cortical and subcortical GM at the region-of-interest level.21 Our study represents the extension of that work to the voxel level and the inclusion of a multivariate analytic approach that incorporated data from multiple discrete cognitive domains.
We hypothesized that cognitively impaired patients with SPMS exhibit decreased cerebral perfusion in functionally relevant brain regions, such as the prefrontal cortex, compared with nonimpaired patients with SPMS, and that these reductions would remain significant in the context of global GM, WM, and WML volumes. Focal (ie, voxelwise) tissue volumes were also explored as predictors of cognitive impairment and cerebral perfusion.
Materials and Methods
Patients
This study was approved by the research ethics boards of Sunnybrook Health Sciences Centre and St. Michael's Hospital. Patients with SPMS were prospectively recruited during a 1-year period from 2 tertiary referral MS clinics. Charts of potential participants were screened by a senior neurologist before recruitment. Exclusion criteria were a history of drug/alcohol abuse, use of disease-modifying drugs or steroids within the past 6 months, premorbid (ie, pre-MS) psychiatric history, head injury with loss of consciousness, concurrent medical diseases (eg, cerebrovascular disease), and MR imaging or contrast agent contraindications. Clinical data included age, sex, education level, and disease duration. MR imaging acquisition, neurologic examination, and Expanded Disability Status Scale assessment were completed on the same day.
Cognitive Testing
The Minimal Assessment of Cognitive Function in Multiple Sclerosis was administered under the supervision of a senior neuropsychiatrist.22 The Minimal Assessment of Cognitive Function in Multiple Sclerosis is a comprehensive and efficient assessment tool consisting of 7 neuropsychological tests: the Paced Auditory Serial Addition Test (working memory/processing speed), Symbol Digit Modalities Test (processing speed), California Verbal Learning Test-II (verbal memory), Brief Visuospatial Memory Test-Revised (visuospatial memory), Delis-Kaplan Executive Function System (executive function), Controlled Word Association Test (verbal fluency), and judgment of line orientation (visuospatial perception). Impairment on an individual test was defined as scoring >1.5 SDs below normative data from healthy controls.23 Patients with ≥2 test impairments were designated as being cognitively impaired.23 Beck Depression Inventory scores were also obtained due to the association between depression and cognitive impairment in patients with MS.10
MR Imaging Acquisition
MR imaging was performed on a 3T scanner (Achieva, Philips Healthcare, Best, the Netherlands) with a 16-channel phased array coil. We acquired the following sequences: 1) turbo field-echo volumetric T1 (TR/TE/flip angle, 9.5 ms/2.3 ms/12°; FOV, 24 cm; matrix size, 256 × 164; section thickness, 1.4 mm); 2) proton attenuation–weighted/T2 (TR/TE/flip angle, 2900 ms/10.7 ms/90°; FOV, 23 cm; matrix, 256 × 261; section thickness, 3 mm); and 3) field-echo EPI DSC (TR/TE/flip angle, 1610 ms/30 ms/60°; FOV, 22 cm; matrix, 128 × 128; in-plane voxel size, 1.7 × 1.7 mm; section thickness, 4 mm; 25 sections; no gap). Ten milliliters of gadobutrol (Gadovist; Bayer Schering Pharma, Toronto, Ontario, Canada) (1 mmol/mL) was administered by an IV pump injector at a rate of 5 mL/s followed by a 25-mL bolus of saline at 5 mL/s. A preloading dose of contrast agent was not necessary because T1 effects are less significant in T2*-weighted sequences, as opposed to T2-weighted sequences. Additionally, these effects do not impact our method of perfusion quantification, which uses steady-state T1 values. A total of 80 field-echo EPI DSC images were acquired at 1.6-second intervals with the contrast agent injection occurring at the 10th image. Inversion recovery Look-Locker-EPI (TR/TE/flip angle, 14.4 ms/7 ms/16°; TI, 15.8 ms; FOV, 22 cm; matrix, 128 × 128; section thickness, 5 mm) was performed immediately before and after DSC imaging.
Image Processing
qCBF and qCBV maps were generated from the DSC and Look-Locker-EPI images by using the bookend technique,17⇓–19 which uses T1-weighted pre- and postgadolinium reference scans before and after DSC imaging. These Look-Locker-EPI bookend scans permit arterial input function–independent DSC calibration by quantifying parenchymal (ie, deep WM) and blood pool (ie, superior sagittal sinus) T1 changes while correcting for intra- to extravascular water exchange by using a water-correction factor.17 Use of the bookend technique in SPMS and comparison of the water-correction factor between patients with SPMS and healthy controls has been previously reported.21 The normalization of these perfusion maps to MNI space and the multispectral tissue segmentation of the structural scans are comprehensively described in the On-Line Appendix.
Statistical and Image Analysis
Clinical data were compared between patients with or without cognitive impairment by using the Wilcoxon rank sum test for continuous variables or the Pearson χ2 test for dichotomous variables. Results for continuous variables were expressed as median (interquartile range) and results for dichotomous variables were expressed as proportions. Analysis of the perfusion maps and segmented tissue volumes with respect to cognitive status by using the mass univariate SPM technique for both and using the multivariate PLS approach for the former is detailed in the On-line Appendix.
Results
Clinical Characteristics
Forty-five patients with SPMS (cognitive impairment, 25/45; 55.6%) were prospectively recruited into this study from 2 tertiary referral MS clinics. The clinical characteristics of these patients, including age, sex, education level, disease duration, Expanded Disability Status Scale score, and Beck Depression Inventory score, were not significantly different between those with and without cognitive impairment (P > .05) (Table 1). There was a trend toward higher Beck Depression Inventory scores in impaired patients (P = .07).
Clinical characteristics of patients with SPMS
Brain and Lesion Volumetry
Significant differences in global tissue volumes were present between cognitively impaired and nonimpaired patients (Table 2). Cognitively impaired patients exhibited lower normalized GM volume (P = .003) and higher normalized WML volume (P = .002) than unimpaired patients. There was no significant difference in normalized WM volume (P > .05) or significant focal GM, WM, or WML volume differences (PFWE > .05).
Global tissue volumes normalized to total intracranial volume
Cerebral Perfusion
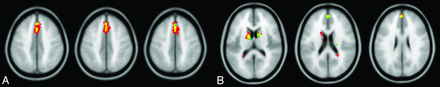
Mass univariate SPM analysis detected a single region of significantly reduced qCBV in cognitively impaired patients with SPMS (PFWE < .05; kE ≥ 20; 479 voxels) (Fig 1A, red and yellow overlays), centered on the superior medial frontal cortex (medial surface of the left and right superior frontal gyri, BA 6). The peak voxel intensity was located at 0,20,46 in MNI space. Significantly decreased qCBV spanned the bilateral superior frontal gyri (BA 6 [110 voxels] and BA 8 [61 voxels]) and bilateral anterior cingulate cortices (BA 32 [67 voxels]). No significant qCBF changes were observed (PFWE > .05).
A, Decreased qCBV detected by SPM and PLS. Inferior-to-superior arrangement of axial sections with 3 different color overlays. The red overlay represents significantly reduced qCBV in cognitively impaired patients with SPMS identified by SPM alone. The green overlay represents significantly reduced qCBV in the same patients identified by PLS alone. The yellow overlay represents the overlap of the SPM and PLS results and thus significantly reduced qCBV found by both analytic techniques. B, Decreased qCBV and qCBF detected by PLS. Inferior-to-superior arrangement of axial sections with 3 different color overlays. The red overlay represents significantly reduced qCBV in cognitively impaired patients with SPMS identified by PLS. The green overlay represents significantly reduced qCBF in the same patients identified by PLS. The yellow overlay represents the overlap of the qCBV and qCBF hypoperfusion.
Multivariate PLS analysis yielded 2 significant LVs based on permutation testing of qCBV maps (LV1: P = .008, cross-block covariance = 52.0%; LV2: P = .004, cross-block covariance = 15.1%). LV1 revealed a more widespread pattern of significantly reduced qCBV in cognitively impaired patients than that found by SPM (bootstrap ratio > 5, kE ≥ 20) (Fig 1A, green and yellow overlays; Table 3), though the PLS-detected region was spatially correlated with the peak of the SPM-detected region. Significantly reduced qCBV in the bilateral superior frontal gyri (BA 6) was identified by both SPM and PLS (Fig 1A, yellow overlay). PLS did not detect significantly reduced qCBV in BAs 8 or 32 as shown by SPM but did identify significantly lower qCBV in BA 9 of the bilateral superior frontal gyri and the bilateral thalami and caudate nuclei.
Regions of decreased qCBV in cognitively impaired patients with SPMS (PLS Analysis)
PLS yielded 2 significant LVs based on permutation testing of qCBF maps (LV1: P = .006, cross-block covariance = 53.1%; LV2: P = .002, cross-block covariance = 15.8%). LV1 demonstrated a medial pattern of significantly reduced qCBF in cognitively impaired patients (bootstrap ratio > 5, kE ≥ 20) (Fig 1B, green and yellow overlays; Table 4). While SPM did not detect significantly decreased qCBF, the PLS clusters of qCBF reduction were spatially correlated with those of qCBV reduction. This concordance was observed in the bilateral superior frontal gyri (BA 9) and the bilateral thalami and caudate nuclei (Fig 1B, yellow overlay).
Regions of decreased qCBF in cognitively impaired patients with SPMS (PLS Analysis)
With the exception of California Verbal Learning Test-II Delayed Recall, all the correlations between predictive variables (including overall patient impairment, individual test impairments, and normalized GM and WML volumes) and the observed patterns of qCBF and qCBV had 95% confidence intervals not overlapping zero (Table 5). The strongest correlations (|r|> 0.5) were shown between qCBF/qCBV and normalized WML volume (r = −0.71/r = −0.72), Symbol Digit Modalities Test (r = −0.56/r = −0.52), Controlled Word Association Test (r = −0.54/r = −0.53); overall patient impairment (r = −0.53/r = −0.51); and Brief Visuospatial Memory Test-Revised Delayed Recall (r = −0.51/r = −0.51) (Table 5).
Correlations between predictive variables and brain scores based on either qCBF or qCBV (PLS Analysis)
Discussion
This is the third study to investigate cerebral perfusion and cognitive status in patients with MS and the second to examine cortical perfusion in this context.20,21 We demonstrated significant superior medial frontal cortex hypoperfusion in cognitively impaired patients with SPMS relative to nonimpaired patients with SPMS via both SPM and PLS. The latter methodology additionally identified significant subcortical GM hypoperfusion in impaired patients. The Symbol Digit Modalities Test, Controlled Word Association Test, Brief Visuospatial Memory Test-Revised Delayed Recall, and overall patient impairment were the neuropsychological outcomes that displayed the strongest correlations with perfusion reduction. While structural analyses did not identify any focal GM, WM, or WML volume differences between cognitive groups, reduced global GM volume and increased global WML volume were shown in impaired patients.
Using SPM and PLS analyses, we demonstrated spatially concordant qCBV reductions in BA 6 of the bilateral superior frontal gyri of cognitively impaired patients. In addition, spatially proximal qCBV reductions were observed in impaired patients in BA 8 (bilateral superior frontal gyri) and BA 32 (bilateral anterior cingulate cortices) by SPM only and in BA 9 (bilateral superior frontal gyri) by PLS only. PLS solely detected decreased subcortical qCBV in the bilateral thalami and caudate nuclei of impaired patients. This technique also revealed qCBF reductions in BA 9 (bilateral superior frontal gyri) and the bilateral thalami and caudate nuclei of impaired patients not observed by SPM. The reductions in qCBF and qCBV revealed by PLS were highly concordant, with only 2 spatially isolated regions of qCBV or qCBF attenuation being detected in BA 6 (bilateral superior frontal gyri) and BA 31 (right posterior cingulate cortex), respectively.
The association between reduced subcortical GM perfusion and impaired cognitive performance observed in our study is in agreement with a prior MS study,20 which in contrast to the present study did not additionally examine cortical perfusion. Previous work by our group demonstrated significantly reduced qCBV in the bilateral medial superior frontal regions of cognitively impaired patients.21 This current study confirmed those results at the voxel level and localized such hypoperfusion to various BAs within the bilateral superior frontal gyri. Prior arterial spin-labeling and PET studies have identified reduced cerebral perfusion and metabolic rates, respectively, in multiple structures of the prefrontal cortex and subcortical GM of patients with MS compared with healthy controls.24,25 Although the MS subtype was not considered in the PET study,24 the arterial spin-labeling study revealed hypoperfusion in patients with benign MS, SPMS, and primary-progressive MS, but not relapsing-remitting MS.25 Such pre-existing diffuse hypoperfusion in the prefrontal cortex and subcortical GM of multiple MS subtypes independent of cognitive impairment, together with the focal hypoperfusion in the superior medial prefrontal cortex, thalami, and caudate nuclei of cognitively impaired patients reported in our study, suggests a progression from generalized to localized hypoperfusion in the clinical context of cognitive impairment.
The single prior study to use voxel-based morphometry to investigate voxelwise brain volumetry and cognition reported that cognitively impaired patients with MS displayed significantly reduced frontal, temporal, and parietal GM volume.26 The study was, however, limited by the lack of normalization for head size and by comparing cognitively impaired patients with MS with healthy controls. Controlling for head size by either including total intracranial volume or total GM volume as a statistical covariate is an essential feature of voxel-based morphometry studies.27 Furthermore, a recent report suggested that comparing cognitively impaired patients with MS to nonimpaired patients with MS is more appropriate than comparing them with healthy controls.5
In our study, normalized WML volume was the single most significant contributor to qCBF and qCBV reduction. An expected inverse relationship between perfusion reduction and increased WML volume was observed by using PLS, though it remains unclear whether increased WML volume primarily reduces perfusion or is the result of prolonged hypoperfusion. Axonal transection is a hallmark of WMLs in MS.28 Similar disruption of WM tracts, such as the superior longitudinal fasciculi, projecting to cortical and subcortical GM could abate neural signaling and precipitate subsequent reductions in neural activity and GM perfusion. Moreover, neurophysiologic observations have suggested that there is an internal tendency for any neural activity to become quiescent in the absence of input.29 The lack of WML volume loss in cognitively impaired patients at the same spatial resolution as the observed hypoperfusion, however, argues against secondary metabolic downregulation due to either axonal loss or attenuated neural activity. Similarly, if brain atrophy is a direct pathologic cause of reduced cerebral perfusion, one might expect GM and WM volumes to exhibit differences on a scale similar to the focal changes in qCBF and qCBV. Alternatively, it is not unreasonable to hypothesize that diminished cerebral perfusion contributes synergistically to WML formation along with other underlying MS pathophysiologies, albeit in a manner generalized with respect to cognitive impairment.30
Information processing speed is the cognitive domain most commonly affected in patients with MS.5⇓⇓–8 The Paced Auditory Serial Addition Test and Symbol Digit Modalities Test are the 2 most frequently administered measures of processing speed in MS studies and are both included in the Minimal Assessment of Cognitive Function in Multiple Sclerosis22 and Brief Repeatable Battery of Neuropsychological Tests.31 Significant differences between patients with MS and healthy controls have been demonstrated for both measures,32⇓⇓⇓⇓–37 but most studies have identified the Symbol Digit Modalities Test as the superior predictor.33⇓⇓⇓–37 The Symbol Digit Modalities Test is considered a purer measure of processing speed compared with the Paced Auditory Serial Addition Test, which has been recently shown to assess both processing speed and working memory.35,38
Traumatic brain injury studies have shown that lesions impinging the superior medial frontal cortex result in impairments in energization, a putative process conceptualized as controlling the initiation and sustaining of any response.29 Patients with lesions disrupting the bilateral superior medial frontal lobes exhibited slowed reaction times on neuropsychological tests.39,40 Specifically, lesions in BAs 6, 9, 24, and 32 were significantly associated with prolonged reaction times.40 These findings are concordant with our observed bilateral superior medial frontal qCBV deficits in cognitively impaired patients with SPMS localized to BAs 6, 8, and 32 by SPM and BAs 6 and 9 by PLS. Furthermore, PLS demonstrated attenuated bilateral qCBF in BA 9. The spatial similarity between these perfusion deficits and the traumatic brain injury lesion sites intimates that patients with SPMS may also experience impaired reaction time. It is, therefore, highly significant that the Symbol Digit Modalities Test, a pure measure of processing speed, was the neuropsychological test most strongly correlating with cerebral perfusion in our study.
Potential limitations of the present study include the lack of complete overlap between the SPM and PLS qCBV results. The discrepancy likely reflects methodologic differences between the 2 analytic techniques. PLS is designed to detect potentially subtle or complex interdependencies between voxel intensities and behavioral performance. Decomposition of the correlation matrix of voxel and behavioral data enables the detection of spatially distributed patterns involving potentially distal voxels and multiple behavioral outcomes. This multivariate approach contrasts with the mass univariate approach of SPM, which compares mean values between coordinate voxels from 2 dichotomized patient groups and thus detects spatially localized patterns. Despite these technical differences, hypoperfusion localized to the bilateral superior medial frontal cortices in both the SPM and PLS analyses highlights the robust nature of these deficits.
Another potential limitation is the relatively modest sample size, but this cohort represents the largest group of patients with MS studied to assess the relationship between perfusion and cognition. A final potential limitation is the necessity for contrast agent administration for DSC acquisition. This constraint will result in a minority of patients with MS not being able to undergo this procedure due to renal impairment or other contrast agent contraindications, but these few exclusions are offset by the pervasive use of DSC imaging in the clinical setting. There was a trend toward a higher severity of depressive symptoms in cognitively impaired patients. Although this did not reach significance, depression is associated with poor cognitive performance in patients with MS.10
Conclusions
This study identified hypoperfusion in the superior medial frontal cortex and subcortical GM of cognitively impaired patients with SPMS compared with nonimpaired patients with SPMS. These findings correlated most strongly with performance on the Symbol Digit Modalities Test, a sensitive marker of processing speed commonly impaired in MS.
Footnotes
Disclosures: Liesly Lee—UNRELATED: Consultancy: consultant to different pharmaceutical companies involved with multiple sclerosis, but not with the submitted manuscript, Payment for Lectures (including service on Speakers Bureaus): lectures on the topic of multiple sclerosis, but not related to the content of the article, Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: funded for travel to meetings by pharmaceutical companies for MS-related activities, but not related to the content of the article, Other: received funding from consultancy services, Speaker Bureaus, clinical trials involved with multiple sclerosis, but not related to content of article (companies include Biogen Canada, Serono Canada, Teva Neurosciences, Schering, BioMS, Bayer Canada, Novartis Canada, Sanofi-Aventis, Allergan). Timothy Carroll—RELATED: Grant: National Institutes of Health, Comments: Much of the development was based on a National Institutes of Health R01, UNRELATED: Other: United States Patent and Trademark Office, Comments: Dr. Carroll's group at Northwestern University has been awarded a patent on a similar technology. To date, we have not received any royalties or licensing on this patent. Anthony Feinstein—UNRELATED: Grants/Grants Pending: MS Society of Canada,* Payment for Lectures (including service on Speakers Bureaus): Merck-Serono, Teva, Biogen, Royalties: Cambridge University Press, Johns Hopkins University Press. *Money paid to the institution.
This study was supported by the Physician Services Incorporated Foundation and the Multiple Sclerosis Scientific Research Foundation.
The authors declare no conflict of interest.
References
- Received February 1, 2012.
- Accepted after revision March 9, 2012.
- © 2013 by American Journal of Neuroradiology