Abstract
SUMMARY: Hepatocerebral MPV17-MDS is quite rare (<30 confirmed cases), with limited findings described on MR imaging. We report 2 siblings having abnormalities within the reticular formation of the lower brain stem and within the reticulospinal tracts at the cervicocranial junction on T2WI. The presence of these MR imaging findings (relative to previous reports) raises the possibility that they represent subtle but characteristic findings corresponding to clinically observed abnormalities of tone encountered with this recently described disorder.
ABBREVIATIONS:
- 1H-MR spectroscopy
- proton MR spectroscopy
- MDS
- mitochondrial depletion syndrome
- MPV17-MDS
- MPV17-related mitochondrial depletion syndrome
- T1WI
- T1-weighted imaging
- T2WI
- T2-weighted imaging
MPV17-related hepatocerebral MDS is a rare congenital autosomal recessive disorder typically characterized by hepatic failure, failure to thrive, and neurologic findings (dependent on the age of onset), such as hypotonia and dystonic movements.1,2 It is 1 of several described MDSs recently confirmed by dedicated laboratory and genetic testing.2 Presentation usually occurs in the first months of life, with the life span typically limited to months.1,2 The MR imaging literature describing MPV17-MDS is sparse and nonspecific, but the variable findings that have been reported range from normal-to-diffuse white matter abnormalities, which may resemble a leukodystrophy or hypomyelination.1 Particular regions noted in case reports include the cerebellar white matter, middle cerebellar peduncles, substantia nigra, and a single patient with subtle involvement of the dorsal brain stem.2⇓–4 Herein, we describe 2 infant siblings with MPV17-MDS exhibiting nearly identical involvement on T2WI at the cervicomedullary junction and within the lower dorsal brain stem.
Case Reports
Case 1
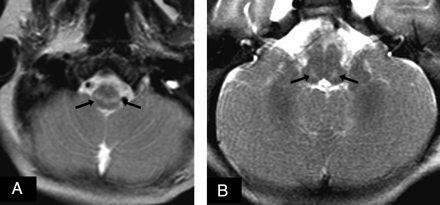
A 3-month-old male infant with no significant perinatal/neonatal history presented with progressive deficiencies in feeding, jaundice, failure to thrive, and mild hypotonia; elevated liver enzymes were subsequently detected. Thereafter, a 1.5T MR imaging at 5 months of age demonstrated mild moderately delayed myelination, as represented on T1WI by limited hyperintensity within the anterior limb of the internal capsule, the corpus callosum splenium and lack of significant occipital white matter hyperintensity. There were hyperintense abnormalities on T2WI bilaterally within the reticular formation of the lower dorsal brain stem and the reticulospinal tracts of the cervicomedullary junction (Fig 1), without reduced diffusion. Findings of point-resolved 1H-MR spectroscopy of the basal ganglia and periventricular white matter appeared normal for his age, without a lactate peak and with equivalent N-acetylaspartate and choline peaks. A head CT at 6.5 months of age did not demonstrate any significant abnormalities or overt atrophy. Hepatomegaly was clinically apparent and confirmed by CT. Muscle biopsies were performed but were negative for the more common mitochondrial disorders. The patient died at 7 months of age due to hepatic failure. Liver necropsy was nondiagnostic due to inadequate samples. The parents subsequently had 2 developmentally normal girls.
At 5 months of age, the male sibling had mild moderately delayed myelination on T1WI (not shown). A and B, There was abnormal T2 hyperintensity (arrows) extending from the reticulospinal tracts at the cervicomedullary junction up to the reticular formation of the medulla and pons, without reduced diffusion on diffusion-weighted imaging (not shown). 1H-MR spectroscopy (not shown) did not demonstrate a lactate peak.
Case 2
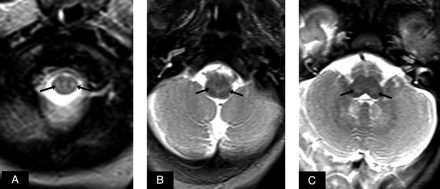
Approximately 2 years following the second developmentally normal girl, a daughter was born with no significant perinatal/neonatal concerns but presented at 4–5 weeks of age with liver dysfunction, jaundice, mild hypotonia, and failure to thrive. At this time, an MR imaging demonstrated myelination appropriate for her age on T1WI, and the MR imaging findings were initially interpreted as negative. Nevertheless, retrospective review (after obtaining and comparing with the first infant's images) demonstrated mild T2 hyperintensity of the dorsal lower brain stem within the reticular formation and within the reticulospinal tracts of the cervicomedullary junction, without reduced diffusion (Fig 2). 1H-MR spectroscopy was not performed. A muscle biopsy was negative for the more common mitochondrial disorders, and an extensive blood work-up was negative for leukodystrophies. A liver biopsy was considered nondiagnostic due to inadequate samples. The patient died at 4 months of age. A liver postmortem examination with samples evaluated by quantitative real-time polymerase chain reaction confirmed mitochondrial DNA copy number at 16% of the expected value for her age, and MPV17 gene sequencing analysis was positive for 2 heterozygous mutations.
At 5 weeks of age, the female sibling did not have overt hypomyelination on T1WI (not shown). A–C, However, there was abnormal hyperintensity on T2WI (arrows), extending from the reticulospinal tracts at the cervicomedullary junction up to the reticular formation of the medulla and pons in a distribution similar to that of her sibling.
Discussion
Spinazzola et al (2006)5 first described the mutation in the nuclear MPV17 gene, which leads to a defective inner mitochondrial membrane protein of unknown function and subsequent mitochondrial DNA depletion. At this time, there have been fewer than 30 patients described with confirmed MPV17-MDS.1,2 Because the clinical findings can be variable and nonspecific for MDS, there must be a suspicion for this disease to initiate the dedicated genetic testing that is necessary for diagnosis; therefore, characteristic MR imaging findings that could alert a clinician to this disorder would have diagnostic utility.
Although the reticular formation is a poorly defined area on MR imaging, it is associated with major subnetworks, including the reticulospinal tracts and descending reticular activating system, which are responsible for tone, posture, and balance and have connections with the cerebellum, which is implicated in this disorder.1,6 Lesions involving such locations may correspond with the typical late neurologic presentation of MPV17-MDS, which often involves hypotonia or dystonic movements.1,2 In the limited available reports of MPV17-MDS, the MR imaging findings have been described as normal (most commonly), “nonspecific white matter abnormalities,” “cytotoxic edema” involving the deep and subcortical white matter of the cerebrum, or “atrophy,” with only a few reports of specific regional abnormalities.1,3⇓–5,7 We reviewed the available images in these clinical reports and found only 1 with dorsal brain stem involvement, which we opine is localized within the reticular formation.3 Thus, the incidence of involvement of the reticular formation or reticulospinal tracts in MPV17-MDS is unknown.
Our description of 2 siblings with nearly identical involvement on MR imaging of the reticulospinal tracts and reticular formation, in conjunction with abnormalities in muscle tone, is most likely consistent with insult to these locations. The findings suggest that subtle but characteristic MR imaging findings could exist in MPV17-MDS. From a technical standpoint, these findings are at the caudal portion of the MR image and thus may not always be covered on axial images. In addition, the involved areas may only have subtle hyperintensity in the earlier stages that can be easily overlooked, as in patient 2. Thus, we concur with previous recommendations to use serial imaging to detect progression of subtle abnormalities on MR imaging or potential hypomyelination.3
References
- Received October 26, 2010.
- Accepted after revision November 2, 2010.
- © 2012 by American Journal of Neuroradiology