Abstract
BACKGROUND AND PURPOSE: Palpebral AVMs (pAVMs) are rare vascular lesions for which the treatment is challenging. Our aim was to present the technical aspects of the presurgical treatment by interventional neuroradiology of pAVMs and to report the clinical and angiographic results of combined (interventional neuroradiology/surgery) treatment of these malformations.
MATERIALS AND METHODS: Nine patients (5 females, 4 males) with a mean age of 22 years (range, 12–35 years) were treated in our department from December 1992 to April 2007 for superficial pAVMs. Seven patients presented with isolated pAVMs, while 2 had hemifacial AVMs. Ten TAE procedures, by using a liquid embolic agent (glue or Onyx) or microparticles, were performed in 7 patients. Six patients underwent absolute alcohol, glue, or sclerotic agent injection by direct puncture in 8 procedures. Clinical and angiographic follow-up were performed with a mean delay of, respectively, 6.3 and 5 years.
RESULTS: Three patients had a single EVT. Iterative procedures were performed in 5 patients. In 1 patient, EVT was not performed because of the risk of occlusion of the central retinal artery. No complication occurred except 1 case of transient palpebral hematoma. No visual acuity loss related to an endovascular procedure was reported. Exclusion of the AVMs at the end of the procedure was >75% in all cases and total in 3/8 cases. All the patients except 2 underwent at least 1 surgical procedure after the embolization. Good clinical regression of the mass was obtained in all patients at long-term follow-up.
CONCLUSIONS: Combined endovascular and surgical treatment of pAVMs is an effective and safe technique with good clinical results at long-term follow-up.
ABBREVIATIONS:
- AV
- arteriovenous
- AVF
- arteriovenous fistula
- CRA
- central retinal artery
- DP
- direct puncture
- ECA
- external carotid artery
- EVT
- endovascular treatment
- IMA
- internal maxillary artery
- n-BCA
- n-butyl 2-cyanoacrylate
- OA
- ophthalmic artery
- pAVM
- palpebral arteriovenous malformation
- PVA
- polyvinyl alcohol
- SOV
- superior ophthalmic vein
- TAE
- transarterial embolization
According to the International Society for the Study of Vascular Anomalies, pAVMs are rare facial high-flow superficial vascular malformations.1 Their origin is not well-established, but they seem to be congenital.2,3 They correspond to an abnormal connection between feeding artery branches and dilated draining veins through a net of dysplastic vessels: the so-called nidus.4,5 Superficial AVMs are serious malformations because they are hemodynamically active.6,7 Depending on their size and aggressiveness, treatment is proposed because they may cause hemorrhage and/or may be responsible for deformity.8 The management of such AVMs is challenging because of the close relationship of these malformations with the CRA.9 The high risk of recurrence/progression should also be considered in the management of such superficial AVMs.8
We report our experience of presurgical endovascular/percutaneous management of 9 consecutive patients with pAVMs referred to our institution for treatment.
Materials and Methods
We retrospectively reviewed the charts of 9 consecutive patients (5 female and 4 male, 12–35 years of age; mean, 22 years) treated for angiographically proved pAVMs in our department from December 1992 to April 2007. The AVM was limited to the palpebral area in 7 patients, while 2 others had hemifacial AVMs. All the pAVMs were unilateral. Eight were located on the upper eyelid; only 1 arose from the inferior eyelid. The mean duration from initial clinical symptoms to diagnosis and treatment was 15.1 years (range, neonate to 35 years).
Clinical symptoms were assessed for all patients by both an ophthalmologist and a neuroradiologist, including visual field, visual acuity, oculomotricity, and cutaneous examination of the eyelids.
Four of the 9 patients had a previous surgical intervention (mean delay, 4 years; range, 2–6 years). Two patients had 2 prior surgical treatments. One patient had a previous embolization with PVA microparticles through the IMA.
All patients underwent DSA with selective opacification of both ICAs and ECAs in anteroposterior and lateral views centered on orbital and palpebral areas. DSA was retrospectively reviewed in consensus by 2 neuroradiologists (F.C. and R.B.). Feeding arteries, draining veins, and morphology of the nidus were detailed. Endovascular/percutaneous treatment was performed in 8/9 patients. In 1 patient, EVT failed because of the risk of occlusion of the CRA (patient 1). Seven patients had TAE during 10 procedures, 6 patients were treated by DP in 8 procedures, and 5 patients were treated by both techniques. The venous approach was attempted in 1 patient but failed (patient 8). Three patients had a single procedure, 3 others had 2 procedures, and 3 patients had 3 interventions. For TAE, 8 procedures were performed with n-BCA injection, 1 with Onyx-18 (ev3, Irvine, California), and another with PVA microparticles (Contour, 350–500 μm; Boston Scientific, Fremont, California) through the IMA. Four patients were treated by DP, 5 with absolute alcohol, 3 with n-BCA, and 1 with Ethibloc (Ethicon, Nordersteat, Germany).
Perioperative or postprocedural complications were systematically assessed.
Clinical follow-up was obtained for all patients with a mean delay of 6.3 years. Persistence or recurrence of a pulsatile mass, residual mass size, bleeding episodes, visual acuity, and oculomotricity were assessed by both an ophthalmologist and a neuroradiologist. When there was a doubt about a local recurrence at clinical examination, a Doppler sonography was performed (in 1 case, patient 7). Angiographic follow-up was obtained for 5/9 patients with a mean delay of 5 years.
Neither approval of the institutional review board nor patient informed consent is required by the ethics committee of our institution for retrospective analyses of patients' records and imaging data.
Results
Clinical Data
Clinical data are summarized in Table 1.
Clinical data
Seven patients presented with an isolated pulsatile mass of the eyelid (Schöbinger grade II, Table 2).10 Two patients (patients 8 and 9) experienced hemorrhage (Schöbinger grade III). In 3 patients, the mass appeared or worsened during puberty. In 1 patient (patient 9), the mass worsened after a local trauma. No case of appearance or worsening of the pAVM was noticed during pregnancy.
Schöbinger's classification of superficial AVMs14
No patient presented with cardiac failure related to the AVM.
Palpebral ptosis was observed in 6/9 patients; decreased visual acuity, in 2/9; and pain, in 1/9 patients. Neither limitation in oculomotricity nor chemosis nor proptosis was seen (Table 3).
Summary of clinical ocular symptoms before treatment
Angiographic Data
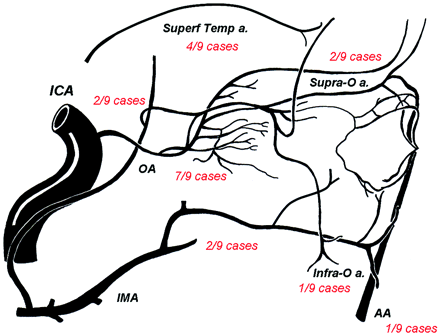
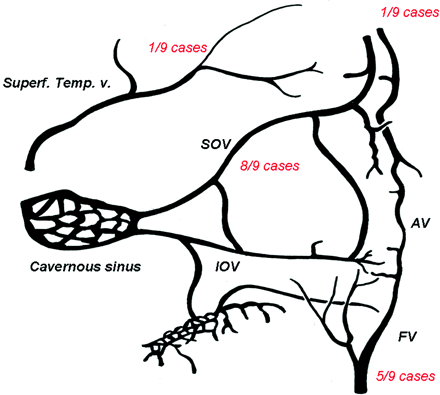
On DSA, detailed review of the angioarchitecture of the pAVMs showed that all the malformations except 1 were fed by palpebral branches arising from the OA (Table 4). In 3 cases, arterial feeders arose from the superficial temporal artery; in 2 cases, from the sphenopalatine; in 2 other cases, from the deep temporal arteries; in 1 case, from the angular artery; and in 1 last case, from the infraorbital artery (Fig 1). Nidus appearance was compact in all cases. In 8 cases, nidus size was inferior to 3 cm; in 1 case, it was superior to 3 cm. Venous drainage was unique in 5 cases and multiple in 4 cases. Draining veins were the SOV in 8 cases, the facial vein in 6 cases, the superficial temporal vein in 1 case and the frontal vein in 1 case (Fig 2). No aneurysm (neither arterial, nidal, nor venous) was observed in the pAVMs. No stenosis on the draining veins was noticed.
Angiographic findings of pAVMs
Schematic representation of the distribution of the main feeding arteries of the pAVMs in our series. For each feeding artery, its frequency in vascularization of the AVM in our series is noted in red. IMA indicates internal maxillary artery; ICA, internal carotid artery; Superf Temp a, superficial temporal artery; Infra-O a, infraorbital artery, OA; ophthalmic artery; AA, angular artery.
Schematic representation of the distribution of main draining veins of the pAVMs in our series. For each draining vein, its frequency in vascularization of the AVM in our series is noted in red. FV indicates facial vein; Superf Temp. V, superficial temporal vein; IOV, Inferior ophthalmic vein; AV, angular vein.
Neuroradiologic Procedures
All data relative to the procedures are summarized in On-line Table 1.
Endovascular procedures were performed presurgically in all patients except 2. For these 2 patients (patients 3 and 8), postembolization surgery was not performed because of the small size of the malformation and a satisfying esthetic result.
Ten TAE procedures by using glue (8/10), particles (1/10), or Onyx-18 (1/10) were performed in 7 patients. Six patients underwent DP in 8 procedures by using absolute alcohol (5/8), n-BCA (2/8), or Ethibloc (1/8) injection. Three patients had a single EVT. Iterative procedures (from 2–3 procedures) were performed in 5 patients. In 1 patient (patient 1), EVT failed because of the risk of potential occlusion of the CRA. In another patient, transvenous embolization also failed (patient 8).
Immediate Angiographic Results
Immediate postembolization angiograms showed complete angiographic exclusion of the pAVMs in 3/8 patients. In the remaining 5 patients, >75% of the AVM nidus was angiographically excluded at the end of the procedure (On-line Table 2).
Postembolization Surgical Treatment
All patients except 2 (patients 3 and 8) underwent a surgical excision of the pAVM performed by ophthalmologists specialized in orbital surgery and oculoplastics, after percutaneous treatment/EVT. Four patients underwent surgery between days 1 and 2; two patients had delayed surgery, respectively, at 1 and 7 months (patients 2 and 9), because despite the small size of the their pAVMs, the sole EVT could not cure the lesion, leading to a residual unesthetic mass observed at early clinical follow-up. Finally, 1 patient had a surgery 8 years after a first attempt of embolization of the pAVM because of a growing mass.
Surgery was performed with the patient under general anesthesia. The peritumoral area was infiltrated by using saline to facilitate the surgical dissection. An incision was made in the superior lid line to the lateral canthus followed by a subcutaneous dissection. The orbicularis muscle was also dissected to expose the vascular malformation. The vascular mass was dissected, and a total excision was performed after coagulation of its feeding pedicles. The levator muscle was then reattached to the superior tarsus with 3 stitches by using Vicryl 5.0 surgical thread (Ethicon, Auneau, France). The orbicularis muscle was sutured with Vicryl 6.0 surgical thread, and the skin was closed by using silk thread 6.0.
Additional surgery for blepharoptosis was performed in 3 patients. In these cases, the levator muscle was reattached to the anterior tarsal surface and the excessive skin was removed when necessary.
Illustrative Case
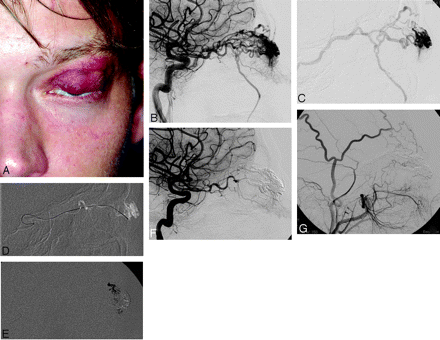
Case 9 (Fig 3).
A 21-year-old man was referred to our institution for the management of a recently appearing mass of the left superior eyelid. The patient reported an episode of eyelid trauma 2 years ago. Clinically, the palpebral mass had a red coloration and was pulsatile (Fig 3A). DSA showed a pAVM located in the medial part of the left superior eyelid, fed, respectively, by palpebral branches from the third segment of the OA (Fig 3B) and by the superficial temporal artery branches (not shown). The nidus was small (25 mm) and compact, with no aneurysm inside. Venous drainage was supported by the SOV, facial vein, and frontal vein (Fig 3C). Microcatheterism of the OA was performed by using a 1.5 UltraFlow microcather (ev3). The microcatheter was positioned in the distal part of the superior palpebral branch (Fig 3D), and intranidal injection of Onyx-18 was performed under subtracted fluoroscopy (Fig 3E). Palpebral feeders from the frontal branch of the superficial temporal artery were also catheterized and embolized with Onyx-18 (not shown).
Illustrative case (patient 9). A 21-year-old patient with a left superior eyelid AVM. A, Photograph, frontal view. B, Left ICA angiogram, lateral view, arterial phase. C, Left ICA angiogram, lateral view, venous phase. D, Selective microcatheterism of the OA. E, Onyx-18 injection through a palpebral branch of the OA and superficial temporal artery (not shown). F, Control left ICA angiogram. G, Control left ECA angiogram.
Immediate postprocedure lateral angiograms of the left ICA and ECA showed a total exclusion of the AVM (Fig 3F, –G). Surgical excision of the pAVM was performed 1 month after the endovascular procedure. At 60-month follow-up, the pAVM had almost totally disappeared clinically (not shown).
Clinical and Angiographic Follow-Up
All data relative to clinical and angiographic follow-up in our series are summarized in On-line Table 2.
No complication occurred during or after the endovascular procedure except 1 transient palpebral hematoma (patient 3). Mean delay for clinical follow-up was 6.3 years (range, 1.5–13 years). Total or quasitotal clinical disappearance of the pAVM was observed in all cases at long-term follow-up. During the clinical follow-up, 2 patients presented with a recurrence, with a mean delay of 6 years (3 and 9 years); a second procedure was performed with a good clinical outcome and without recurrence at long-term follow-up. For 1 patient, the persistence of a remnant at 1 year after the first treatment led to a new treatment by DP with satisfying regression of the mass at 13-year follow-up.
Eyelid functionality was evaluated by ophthalmologists at clinical follow-up and was satisfying in all cases.
Angiographic follow-up was performed in 5/9 patients with a mean delay of 5 years (range, 5 months to 10 years). In all cases (5/5), disappearance or only a small remnant of the nidus was observed.
Discussion
Superficial AVMs: Pathophysiology, Natural History, and Classification
The origin of superficial AVMs, generally, and pAVMs, specifically, has not been clearly established. Some authors hypothesized a congenital origin of these lesions.3 Rare genetic mutations, like ones of the RASA1 gene, have been reported as being responsible for the development of some of superficial AVMs.10 Trauma has been described as a potential cause of the appearance or worsening of superficial AVMs,8 including pAVMs.11 In the series of Warrier et al,12 traumas were noticed in as frequently as 50% of the cases. Nevertheless, in larger series on superficial AVMs, causal effects were found in only 20% of the cases.13 Schöbinger et al14 established a classification based on clinical manifestations and complications of superficial AVMs: Grade I corresponded to a blue plane cutaneous lesion; grade II lesions were pulsatile subcutaneous masses; in grade III, hemorrhage was observed; and finally, grade 4 was characterized by a cardiac repercussion of the AVM (Table 2).
Puberty and pregnancy are other causes in the growth of the pAVMs, suggesting a hormonal influence on the evolution of these malformations.
Palpebral AVMs may be isolated or associated with other facial locations that can lead to hemifacial AVMs. Sometime, a pAVM can be associated with other vascular malformations in the central nervous system. Thus, Wyburn-Masson syndrome, also called Bonnet-Dechaume-Blanc syndrome in the French literature, may affect the eyelid, in association with retinal and cerebral AVMs.15,16
The distinction between pAVMs and orbital AVFs is of tremendous importance. AVFs are direct connections between feeding arteries and draining veins, while pAVMs are arteriovenous shunts associated with a network of dysplastic vessels: the nidus. Palpebral AVFs usually occur in post-traumatic conditions, while pAVMs are congenital and may appear or worsen due to trigger factors (trauma, pregnancy, puberty, and so forth). Clinically, AVFs may exceptionally present as a pulsatile eyelid mass.17 AVFs are less aggressive than pAVMs; pAVMs are present from birth, may remain quiescent for years, and become active with a high increase of the flow.18 Treatment is easier for palpebral AVFs because it can be performed by a sole endovascular embolization, while in pAVMs, EVT, most of time, needs to be associated with surgery and often remains partial, focused on the active part of the lesion.19
The differential diagnosis for pAVM include capillary hemangiomas for neonates and solid tumor or venous varices for adults. Clinically, an orbital varicose vein grows in the Valsalva maneuver, in a prone position, and in crying for babies.20 Usually, Doppler sonography and MR imaging help in the confirmation of the diagnosis.
Angiographic Findings in pAVMs
In our series, 8 of 9 patients had pAVMs fed by ICA branches (palpebral branches from the OA); in 1/9 cases, the pAVM was fed only by branches from ECA; in 6/9 cases, pAVMs were fed by both ICAs and ECAs (Fig 1 and Table 4). The draining vein was, most of the time, the SOV (8/9). More rarely, the facial vein drained the pAVM (4/9). The nidus was small (<3 cm) and had a compact appearance in almost all cases. In our study, neither a nidal nor arterial aneurysm was observed. Nevertheless, an aneurysm located on the arterial feeder has already been described in pAVMs.21
EVT
EVT is challenging in these superficial vascular malformations because of the risk of intraorbital hematoma or occlusion of the CRA by uncontrolled embolic agent injection, potentially responsible for monocular blindness. According to most authors8,13 and our institutional experience, therapeutic abstention should be proposed for quiescent lesions. Thus, treatment should only be performed for nonquiescent lesions (growing lesions or bleeding pAVMs) or in cases of a lesion responsible for a major deformity.8
Embolization cannot, most of time, alone achieve a total cure of the AVM.13 Thus, according to our experience, combined treatment associating endovascular embolization and surgical resection should be proposed, with the main intention of curing, as totally as possible, the pAVM.9,13,14 For small pAVMs, embolization alone could be performed with satisfying results (2 cases in our series). The best delay to surgical resection is 24–48 hours, when additional surgery is recommended. The choice of the embolic agent for the treatment of the pAVMs depends on the habits of the operator and on the angioarchitecture of the pAVM. For small pAVMs, glue should be used as a first choice. Side effects of some embolic agents should also be known: Absolute alcohol presents the risk of cutaneous necrosis and general complications (dose-related)22; black skin coloration could appear after Onyx injection in cases of superficial pAVMs.
Comparison of Our Results with Data in the Literature
Palpebral AVMs are very rare. In a review of 627 orbital tumors, Wright1 found only 3 orbital AVMs. Only single case reports and short series have been previously reported.12,23 To our knowledge, fewer than 50 cases have been reported in the literature.12,24–33 The largest series published in the English literature included 8 patients.12 In this study, 7 of the 8 patients had a surgical excision of the AVM. Preoperative embolization was performed in 3 patients: Two had embolization with PVA particles in the arterial feeders and 1 was presurgically treated by platinum coils. Postoperative follow-up (mean delay, 27 months) in this series showed that 6 patients remained symptom-free and 2 required further procedures. In our series, no significant complication occurred during or after the endovascular procedure. All patients had total or quasitotal clinical regression of the mass at long-term follow-up (mean delay, 6.3 years). Disappearance or only a small remnant of the pAVM was observed at long-term angiographic follow-up in all the cases, when performed (5/5).
Conclusions
In this series, combined endovascular and surgical treatment of pAVMs was safe and effective. All patients had a good clinical outcome at 6 years' mean follow-up. No significant complication was reported.
Acknowledgments
We thank Chrisanthi Basdekidou, MD, for her precious help.
Footnotes
-
Disclosures: Jacques Moret. Consultant: Boston Scientific, ev3.
References
- Received November 17, 2010.
- Accepted after revision April 26, 2011.
- © 2012 by American Journal of Neuroradiology