Abstract
BACKGROUND AND PURPOSE: MR perfusion CBF values can distinguish hypoperfused penumbral tissue likely to infarct from that which is likely to recover. Our aim was to determine if CBF thresholds for tissue infarction depend on the timing of recanalization in patients with acute stroke treated with IAT.
MATERIALS AND METHODS: Twenty-six patients with acute proximal anterior circulation strokes underwent DWI and PWI before IAT. rCBF was obtained in the following areas: 1) C with abnormal DWI, reduced CBF, follow-up infarction; 2) PI with normal DWI, reduced CBF, follow-up infarction and 3) PNI with normal DWI, reduced CBF, normal follow-up. rCBF in tissue reperfused at <6 hours (early recanalizers), in tissue reperfused at >6 hours (late RC), and in NRC was compared.
RESULTS: For C, mean rCBF was 0.13 (SEM, 0.002), 0.29 (0.007), and 0.21 (0.004) for early recanalizers, late recanalizers, and nonrecanalizers, respectively (P < .001, for all comparisons). For PI, mean rCBF was 0.34 (0.006), 0.38 (0.008), and 0.39 (0.005) for early recanalizers, late recanalizers, and nonrecanalizers, respectively (P < .001 for early-versus-late recanalizers and versus nonrecanalizers; P > .05 for late recanalizers versus nonrecanalizers). For PNI, the mean rCBF was 0.38 (0.002), 0.48 (0.003), and 0.48 (0.004) for early recanalizers, late recanalizers, and nonrecanalizers, respectively (P < .001 for early-versus-late recanalizers and nonrecanalizers; P > .05 for late recanalizers versus nonrecanalizers). ROC analyzis demonstrated optimal rCBF thresholds for tissue infarction of 0.27 (sensitivity, 80%; specificity, 87%), 0.44 (sensitivity, 77%; specificity, 75%), and 0.41 (sensitivity, 78%; specificity, 77%) for early recanalizers, late recanalizers, and nonrecanalizers, respectively.
CONCLUSIONS: CBF thresholds for tissue infarction in patients with acute stroke are lower in tissue that is reperfused at earlier time points. This information may be important in selecting patients who might benefit from reperfusion therapy.
Abbreviations
- AIS
- acute ischemic stroke
- AUC
- area under the ROC curve
- C
- infarct core
- CBF
- cerebral blood flow
- CBV
- cerebral blood volume
- DWI
- diffusion-weighted imaging
- FLAIR
- fluid-attenuated inversion recovery
- IAT
- intra-arterial therapy
- ICA
- internal carotid artery
- IV
- intravenous
- MCA
- middle cerebral artery
- MTT
- mean transit time
- OOP
- optimal operating point
- PI
- penumbra that infarcts
- PNI
- penumbra that does not infarct
- PWI
- perfusion-weighted imaging
- rCBF
- relative cerebral blood flow
- ROC
- receiver operating characteristic analysis
- SE
- standard error
- SEM
- standard error of the mean
- Tonset
- time from stroke onset to vessel recanalization
- tPA
- tissue plasminogen activator
To best select candidates for reperfusion therapy, one must identify initial neuroimaging findings that suggest potentially salvageable tissue. Selection criteria for thrombolysis are traditionally based on unenhanced CT findings.1 Patients with intracranial hemorrhage, hypoattenuation in more than one-third of the MCA territory, and intracranial mass are excluded.2 With the advent of advanced MR imaging techniques, there is increasing focus on DWI and PWI. The DWI lesion is generally thought to represent a “core” of tissue destined for infarction.
PWI can measure perfusion parameters including CBV, CBF, and MTT.3 In the early stages of stroke, there are frequently regions characterized by normal diffusion but abnormal perfusion, which are thought to operationally define “ischemic penumbra.”3–6 Previous studies have demonstrated significant differences in CBF values in penumbra that proceeds to infarction (“nonviable penumbra”) versus that which recovers (“viable penumbra”) in patients undergoing conventional therapies.7–9
However, it is unlikely that an absolute CBF threshold for tissue infarction exists. Prior reports have demonstrated that gray matter has a significantly higher MR imaging CBF threshold for tissue infarction than white matter.10,11 The duration of ischemia is also important in determining a CBF tissue infarction threshold. Jones et al12 demonstrated that the CBF threshold for tissue infarction was 10–12 mL/100 g/min for 2–3 hours of occlusion but 17–18 mL/100 g/min for permanent occlusion of the MCA in monkeys.
We hypothesized that acutely ischemic tissue that is successfully reperfused at early time points has lower CBF infarction thresholds than tissue that is reperfused later or not at all. To test this hypothesis in patients with acute stroke treated with IAT, we determined the MR imaging CBF thresholds for tissue infarction, stratified by time to recanalization.
Materials and Methods
Patient Selection
This study was approved by our institutional review board and was Health Insurance Portability and Accountability Act–compliant. Patients with AIS treated with IAT between January 2005 and August 2007 were considered for analysis. Further selection criteria included the following: 1) angiographically proved ICA terminus and/or MCA M1 and/or M2 occlusions, 2) pretreatment DWI and PWI MR studies, and 3) availability of follow-up CT or MR imaging studies at least 24 hours postictus for delineation of final infarct extent.
Imaging Protocols
MR imaging examinations were performed on a 1.5T whole-body scanner (Signa Horizon LX; GE Healthcare, Milwaukee, Wisconsin). DWI was performed by using a single-shot echo-planar spin-echo sequence with two 180° radio-frequency pulses to minimize eddy current warping. For 21 months, 5 images/section were acquired at b=0 s/mm2, followed by 5 at b=1000 s/mm2 in 6 directions, for a total of 35 images/section. For the remaining 11 months, 3 images/section were acquired at b=0 s/mm2, followed by 1 image/section at b=1000 s/mm2 in 25 directions, for a total of 28 images/section. Twenty-three to 27 sections covered the entire brain. Imaging parameters were the following: TR/TE, 5000/80–110 ms; FOV, 22 cm; matrix, 128 × 128 zero-filled to 256 × 256; section thickness, 5 mm; and 1 mm gap.
PWI was performed by using a dynamic susceptibility technique. Serial echo-planar gradient-echo images were acquired with TR/TE, 1500/40 ms; FOV, 22 cm; matrix, 128 × 128; section thickness, 5 mm with a 1-mm gap. Fourteen to 16 section volumes were acquired every 1.5 seconds, and 46–80 such volumes were acquired, for a total imaging time of 69–120 seconds. Ten seconds after image acquisition began, 20 mL of gadopentetate dimeglumine, 0.5 mmol/mL (Magnevist; Bayer Healthcare Pharmaceuticals, Montville, New Jersey) was injected via a peripheral intravenous catheter at 5 mL/s by using a power injector (EnVision CT Injector; Medrad, Indianola, Pennsylvania), followed by a 20 mL of normal saline bolus injected at 5 mL/s.
Signal intensity–versus-time curves for each pixel were converted to concentration-versus-time curves, which were integrated to yield maps of CBV. CBF was calculated by singular-value-decomposition deconvolution.13 A global arterial input function was derived from the MCA ipsilateral to each patient's infarct. MTT was calculated by dividing CBV by CBF.
Unenhanced CT was performed on a multidetector helical scanner (LightSpeed, GE Healthcare) with 5-mm contiguous axial images, 140 kV[peak], 170 mA, and 1-second rotation.
Axial FLAIR images were obtained with TR/TE/TI, 10,002/141/2200 ms; FOV, 24 mm; matrix, 256 × 256 voxels; section thickness, 5 mm with a 1-mm gap; and 1 signal-intensity average.
Image Analysis
The DWI images were already coregistered with the CBF images because they were acquired during the same MR imaging scanning session with the same section thickness. Using a semiautomatic coregistration technique (Analyze 8.0; Mayo Clinic, Rochester, Minnesota), we aligned the follow-up CT or FLAIR images with the initial CBF maps. Briefly, the coregistration algorithm is based on the “normalized mutual information” formulation of Studholme et al.14 In our analysis, the CBF maps were the “Base” and the CT/FLAIR images were the “Match.” The software applies 3D rigid-body transformations of the Match data, fuses the Match and Base data, and calculates an entropy term known as “normalized mutual information” for each iterative step. The software uses a nonlinear optimization algorithm to search for the best registration. Following automated voxel registration, we reviewed the resulting transformed images and determined the quality of each coregistration. In cases in which the coregistration was suboptimal due to edema, tissue loss, or patient motion, we used the manual function in the Analyze software to adjust the Match images so that the contours of the brain and ventricles were aligned.
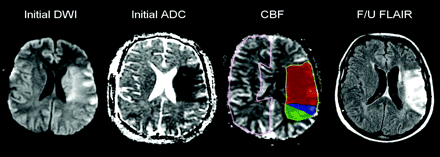
Lesion outlines were created manually by 2 medical students (L.V., K.M.) and corrected by 2 neuroradiologists by consensus (P.W.S., A.J.Y.) (Fig 1). Outlines were drawn around DWI hyperintense/apparent diffusion coefficient hypointense regions on the coregistered DWI images, around hypointense regions on the coregistered CBF maps, around hypoattenuated regions on the coregistered follow-up CT images, and around hyperintense regions on the coregistered follow-up FLAIR images. All segmented volumes were obtained in a blinded manner without comparison among sequences or with clinical data. The outlines were all superimposed on the coregistered CBF maps. Ischemic tissue was then divided into 3 regions on the CBF maps: 1) C with abnormal DWI, reduced CBF, and follow-up infarction; 2) PI with normal DWI, reduced CBF, and follow-up infarction; and 3) PNI with normal DWI, reduced CBF, and normal follow-up images. Tissue in the entire contralateral hemisphere was outlined to provide normalization for the absolute CBF values.
Acute left MCA infarction. Ischemic tissue divided into 3 regions: 1) C (red) with DWI hyperintensity, CBF hypointensity, follow-up infarction; 2) PI (blue) with normal DWI, CBF hypointensity, follow-up infarction; 3) PNI (green) with CBF hypointensity, but normal DWI and follow-up images. Normal tissue in the contralateral hemisphere (pink) was outlined to provide normalization.
Conventional Angiogram Evaluation
Cerebral angiograms following treatment were analyzed by using the Mori scale: grade 0, complete occlusion; 1, distal movement of thrombus without reperfusion; 2, partial recanalization with reperfusion in <50% of the ischemic area; 3, partial recanalization with >50% reperfusion; and 4, complete recanalization/reperfusion.15,16 This scale has been validated for large-vessel occlusions treated with IAT.16 Patients with Mori grades 3 and 4 were considered recanalizers. Patients with Mori grades 0 and 1 were considered nonrecanalizers. For patients with Mori grade 2, we compared their lesion outlines with the angiographic study. Brain regions associated with a recanalized vessel were assigned to the recanalizers group. Brain regions associated with a vessel that remained occluded were assigned to the nonrecanalizers group.
Data Analysis
Normalized rCBF values were produced by dividing the absolute CBF voxel values by the global mean CBF of the contralateral hemisphere in each patient.
The recanalizer group comprised voxel-level CBF values in all sections of patients with Mori 3 and 4, as well as angiographically determined reperfused sections in patients with Mori 2. The nonrecanalizers group comprised voxels in all Mori 0 and 1 sections, as well as nonrevascularized sections in patients with Mori 2. The recanalizers voxels were further subdivided according to patient symptom onset–to-recanalization time. Patients with vessel recanalization earlier than 6 hours were considered early RC. Those recanalized after 6 hours were considered late recanalizers. We chose 6 hours as an arbitrary cutoff because 6 hours is the only time point used in prior studies that has proved benefit of treatment with IAT.17 We did not make further divisions due to our relatively small patient cohort.
Pooled-data ROC was performed at the voxel level to estimate the rCBF thresholds for predicting final infarction. For each group (the early recanalizers, late recanalizers, and nonrecanalizers), voxels with abnormal CBF were pooled into those that were characterized by infarction on follow-up (from the C and PI) and those that were not characterized by infarction on follow-up (from the PNI). The AUC was calculated by using trapezoid approximation, and its SE was calculated by using an approximation method published elsewhere.18 We adopted 3 definitions of a clinically meaningful threshold: the OOP of the ROC curve19; the threshold value with 90% sensitivity; and the threshold value with 90% specificity.
Statistical Analysis
Comparison across study groups was performed by using the Fisher exact test for categoric variables, 1-way analysis of variance for normally distributed continuous variables, and the Kruskal-Wallis test for ordinal or non-normally distributed data. Normality was tested by using the Kolmogorov-Smirnov test. Statistical analysis was performed by using MedCalc for Windows (MedCalc Software, Mariakerke, Belgium). For each group of voxel data, the mean and SEM were calculated. ROCs were performed on a freely distributed statistical platform, R Project version 2.8.1 (R Foundation for Statistical Computing, Vienna, Austria). P < .05 was considered statistically significant.
Results
Demographic and clinical data are presented in Table 1. There were 26 patients (12 men, 14 women) with a mean age of 69.3 ± 17.4 years (range, 24–92 years). Median National Institutes of Health Stroke Scale score on initial examination was 18 (interquartile range, 15–21). Initial angiography identified 13 terminal ICA occlusions, 11 M1 occlusions, and 2 proximal M2 occlusions.
Patient demographicsa
Ten patients received IV tPA before IAT, which included varying combinations of urokinase/tPA injections, angioplasty, and Merci retriever (Concentric Medical, Mountain View, California) and wire manipulation. Eight patients had complete-to-near-complete recanalization (Mori 3 and 4), 10 had partial recanalization (Mori 2), and 8 had no recanalization (Mori 0 and 1). Of the 18 patients who underwent reperfusion, 6 had early (within 6 hours) recanalization, and 12 patients experienced recanalization beyond 6 hours of stroke onset. Comparison of the demographic and clinical variables between early RC, late RC, and NRC is illustrated in Table 1. The follow-up imaging technique was CT in 46% (12/26) and MR imaging in 54% (14/26).
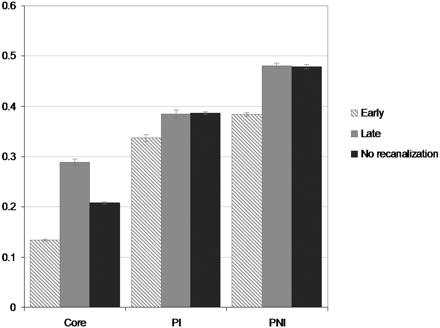
Mean and SEM of rCBF are shown in Fig 2. For core tissue, mean rCBF was 0.13 (SEM, 0.002) for the early recanalizers; 0.29 (0.007) for the late recanalizers; and 0.21 (0.004) for the nonrecanalizers groups, respectively (P < .001, for all comparisons). For PI tissue, the mean rCBF was 0.34 (SEM, 0.006) for the early recanalizers; 0.38 (0.008) for the late recanalizers; and 0.38 (0.005) for the nonrecanalizers groups, respectively (P < .001 for early-versus-late recanalizers and for early recanalizers versus nonrecanalizers; P > .05 for late recanalizers versus nonrecanalizers). For PNI tissue, mean rCBF was 0.38 (SEM, 0.002) for the early recanalizers; 0.48 (0.003) for the late recanalizers; and 0.48 (0.004) for the nonrecanalizers, respectively (P < .001 for early-versus-late recanalizers and for early recanalizers versus nonrecanalizers; P > .05 for late recanalizers versus nonrecanalizers).
Relative CBF (mean, SEM) in ischemic penumbra grouped by final infarct outcome. Each voxel of ischemic tissue was scaled to the global contralateral mean CBF. Differences between early and late recanalizers and early recanalizers and nonrecanalizers in each region were statistically significant (P < .001). Total voxel volumes for C were 301.8, 116.3, and 437.2 cm3 for early recanalizers, late recanalizers, and nonrecanalizers, respectively. For PI, they were 87.4, 85.3, and 505.7 cm3 for early recanalizers, late recanalizers, and nonrecanalizers, respectively; and for PNI, they were 363.3, 344.3, and 545.3 cm3 for the early recanalizers, late recanalizers, and nonrecanalizers, respectively.
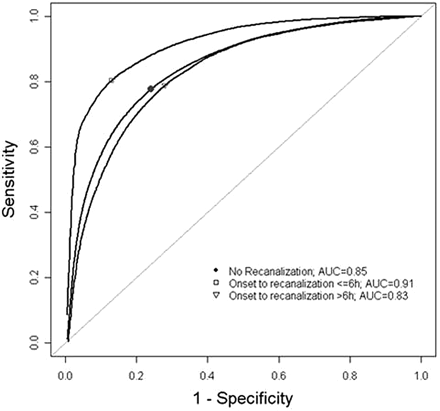
ROC analysis for early recanalizers, late recanalizers, and nonrecanalizers is presented in Fig 3 and Table 2. The AUCs were 0.91 (SE, 0.001), 0.83 (SE, 0.002), and 0.85 (SE, 0.001), respectively. The OOPs for early recanalizers, late recanalizers, and nonrecanalizers were 0.27 (sensitivity, 80%; specificity, 87%), 0.44 (sensitivity, 77%; specificity, 75%), and 0.41 (sensitivity, 78%; specificity, 77%), respectively. The rCBF threshold values to distinguish final infarction with 90% sensitivity were 0.40, 0.63, and 0.60, respectively. The rCBF threshold values to distinguish final infarction with 90% specificity were 0.24, 0.28, and 0.28, respectively.
ROC curves for pooled-voxel rCBF data. The AUC corresponds to the discriminatory power of rCBF in predicting final infarct. The markers indicate optimal operating points, where the tangent line to the ROC curve has slope = 1.
ROC of relative CBF thresholds for acute ischemic tissue viability
Discussion
We have demonstrated that in patients with AIS with anterior-circulation large-vessel occlusion treated with IAT, the CBF threshold for tissue infarction depends on the timing of reperfusion. Specifically, brain tissue supplied by arteries successfully recanalized within 6 hours of stroke onset has a significantly lower rCBF threshold for tissue infarction compared with tissue that is reperfused beyond 6 hours or not reperfused at all. The optimal relative CBF thresholds for infarction in tissue that is reperfused early (<6 hours), reperfused late (>6 hours), and not reperfused are 0.27, 0.44, and 0.41, respectively.
Our results are consistent with animal studies demonstrating the importance of both the duration and severity of ischemia on infarct evolution. In a primate stroke model, Jones et al12 demonstrated that the CBF threshold for tissue infarction was 10–12 mL/100 g/min for 2–3 hours of occlusion, but 17–18 mL/100 g/min for permanent occlusion of the MCA. Multiplying our relative CBF values for infarction (at the OOP) by the mean CBF in humans (50 mL/100 g/min)20 yields similar values of 13.5 mL/100 g/min for early reperfused brain and 20 mL/100 g/min for late reperfused/nonreperfused brain.
The current study builds on previous work by demonstrating the time dependence of CBF thresholds in human stroke. In one study, CT perfusion analysis of 14 patients who underwent IAT demonstrated significantly lower CBF in surviving penumbral white matter in patients with substantial recanalization (Mori grades 3–4; CBF, 9.18 mL/100 g/min) versus patients with little to no recanalization (Mori grades 0–2; CBF, 17.46 mL/100 g/min).21 Among studies examining MR perfusion thresholds,3,6–9,22,23 none have documented a well-defined duration of ischemia. By focusing on patients receiving IAT, we were able to assess the degree and timing of reperfusion reliably. Shih et al22 also studied patients undergoing IAT; however, they did not analyze their results by duration of ischemia.
A novel finding in this study is that with respect to tissue fate, reperfusion after a certain time point appears equivalent to no reperfusion. There was no difference in the mean rCBF for late recanalizers (>6 hours) versus nonrecanalizers in both the PI (mean rCBF = 0.38 and 0.39, respectively) and the PNI (mean rCBF for both = 0.48). This correlates with the finding by Jones et al12 that the CBF threshold for tissue infarction in acutely ischemic monkey brain tissue rises with increasing occlusion time until 3–4 hours and, thereafter, is constant at 17 mL/100 g/min. Furthermore, it has been estimated that the average time interval from onset to completion of a large-vessel stroke is approximately 10 hours.24 The mean time to reperfusion for the late recanalizers was 9.0 hours.
These findings provide the groundwork for creating a time-dependent model of tissue viability based on quantitative cerebral perfusion that would delineate tissue at risk for infarction based on time from stroke onset. Currently, MR imaging–based treatment selection is limited by multiple problems, including the lack of a standardized definition of PWI/DWI mismatch and the paucity of outcome data validating this approach. The selection criterion most often used is a PWI/DWI mismatch ratio of ≥1.2.25 The problem with this criterion is its lack of discrimination because most patients with a large-vessel occlusion have such a mismatch.26 Furthermore, the perfusion parameter most often used to delineate hypoperfusion is MTT, which overestimates tissue at risk.8,23,27
Identifying tissue at risk on the basis of perfusion impairment requires knowledge of 2 important CBF thresholds: 1) the lower threshold that differentiates the infarct core from viable penumbra, and 2) the upper threshold that differentiates at-risk tissue from tissue with benign oligemia. The upper CBF threshold is derived from the ROC curve for nonreperfused tissue. By definition, nonrescued tissue that remains viable is in the region of benign oligemia. As Heiss et al28 demonstrated, a clinically relevant threshold is the 90% sensitivity threshold, with 90% of voxels that ultimately infarct having rCBF values below the threshold. In this study, the 90% sensitivity threshold of the upper boundary yielded an rCBF value of 0.60. This value is in agreement with the study by Rohl et al,7 which assessed patients with untreated stroke who underwent DWI and PWI within 6 hours of onset. If one assumes that most of their patients did not undergo spontaneous recanalization, their rCBF value of 0.59 (sensitivity, 91%; specificity, 73%) represents the upper boundary of the penumbra.
One could argue that knowing the lower CBF threshold discriminating infarct core from salvageable penumbra is not necessary because the core is best identified with DWI and reversibility of the DWI lesion is rare.29,30 However, the time interval from pretreatment imaging to reperfusion with IAT may be several hours. During this time, the initial infarct core extends into penumbra at a rate determined by the degree of hypoperfusion (degree of collateral circulation). Knowing the lower CBF boundary that distinguishes infarct from penumbra at any time point would be helpful to assess what tissue remains at risk during the course of endovascular therapy, thus facilitating the decision to abort versus proceed.
The most clinically relevant threshold for the lower boundary is the 90% specificity threshold because this would maximize the amount of potentially salvageable tissue (90% of voxels that were ultimately rescued had rCBF values greater than the threshold). Due to our small number of patients, we were only able to resolve this time-dependence on the basis of reperfusion within-versus-beyond 6 hours from stroke onset. The 90% specificity thresholds for early reperfusion (≤6 hours) and for late reperfusion (>6 hours) were 0.24 and 0.28, respectively. Practically speaking, time intervals of 30–60 minutes from stroke onset should be the goal of future studies.
The normalized voxel-based analysis used in this study has important advantages.31 Normalization of CBF with the mean value of the contralateral normal brain serves to reduce intersubject variability. Also, grouping data from a large number of voxels enhances statistical power and allows detection of treatment effects in a relatively small patient sample. Our choice of CBF as the perfusion parameter for assessing tissue viability is based on previous work demonstrating its greater accuracy over CBV and MTT in discriminating tissue fate.7,32
The study groups in this analysis were largely well-matched. The significant differences in the degree of recanalization and time to recanalization/procedure termination reflected how these groups were defined. With respect to IV tPA administration, most patients in the early recanalizers group (83%) and a minority of patients in the late recanalizers group (8%) were treated because patients who recanalized earlier were more likely to present within the 3-hour window for IV tPA, as evidenced by the differences in time from onset to imaging. The other notable difference was in the time from onset to imaging, which may explain the differences in mean CBF values for infarct core. In patients who are imaged earlier, there would be less recruitment of the penumbra into the infarct core and, therefore, lower CBF. Other potential factors that could explain this discrepancy are differences in transient reperfusion into the infarct core, inaccuracies in segmentation due to edematous changes or tissue loss distorting anatomy on follow-up images, and inaccuracies in recorded time of stroke onset.
The limitations of this study are primarily related to its retrospective design, as well as to the small patient number (which limited our ability to divide our analysis into smaller treatment time ranges and perform separate analyses of small-versus-large initial strokes) and different treatment regimens. In addition, our CBF maps were created with the most commonly used deconvolution algorithm, which produces lower CBF measurements in regions of tracer-arrival delay. Newer algorithms that are not sensitive to delay33 are now gaining increasing use and could result in CBF thresholds for viability that are higher than those reported in this and previous studies. Furthermore, the utility of the quantitative ischemic thresholds have not been proven in the clinical setting. In the future, if we develop a large historic data base of quantitative thresholds with corresponding clinical factors, we might be able to select an appropriate ischemic threshold for any patient with acute stroke presenting in the emergency department. This would allow us to predict how much of the presenting penumbra would recover with and without treatment and would guide our clinical decision to pursue IAT. Nevertheless, this study demonstrates the feasibility of creating a time-dependent model of tissue viability on the basis of quantitative MR perfusion imaging.
Conclusions
By studying patients with large-vessel stroke with pretreatment MR perfusion imaging and subsequent endovascular therapy, we have demonstrated the time dependence of perfusion thresholds that predict tissue fate in human stroke. We propose a quantitative perfusion-based definition of the penumbra that will incorporate both the degree and duration of perfusion impairment. Further studies are needed to improve the time resolution of these perfusion thresholds.
Footnotes
A.J.Y. is the co-first author.
-
Indicates Fellows' Journal Club selection
-

-
Previously presented in part at: Annual Meeting of the American Society of Neuroradiology, May 31-June 5, 2008; New Orleans, Louisiana.
References
- Received May 3, 2010.
- Accepted after revision August 22, 2010.
- Copyright © American Society of Neuroradiology