Abstract
BACKGROUND AND PURPOSE: MTS is characterized by gliosis and atrophy of the hippocampus and related limbic structures. However, the damage is not limited to those structures with atrophy and has been reported in extratemporal regions. Because volumetric studies are nonspecific, the pathophysiology of the brain damage remains to be solved. MTI is an MR imaging technique more sensitive to subtle neuropathologic changes than conventional MR imaging. Here we combined MTI with VBM analysis to evaluate extratemporal damage in patients with TLE.
MATERIALS AND METHODS: We studied 23 healthy controls and 21 patients with TLE with mean ages, respectively, of 37.6 ± 10.9 and 38.6 ± 9.02 years. All subjects had a full clinical follow-up and MR imaging. We processed the images with VBM for volumetric analysis of WM and GM, as well as with voxel-based analysis of MTR for macromolecular integrity analysis.
RESULTS: In addition to MTR decrease in the temporal lobes, we found a significant decrease in GM and WM volumes. In the WM, the MTR decrease was correlated to volume loss detected by VBM, indicating that brain atrophy may explain part of the MTR decrease. We also found areas in which the MTR decrease was not associated with volume loss, suggesting an additional pathophysiologic process other than neuronal loss and atrophy underlying the MTR changes.
CONCLUSIONS: Our results support the hypothesis that there are widespread lesions in the brain, including the corpus callosum and the frontal lobe, affecting both GM and WM.
Abbreviations
- CNS
- central nervous system
- FDR
- false discovery rate
- GM
- gray matter
- HA
- hippocampal atrophy
- MS
- multiple sclerosis
- MTI
- magnetization transfer imaging
- MTR
- magnetization transfer ratio
- MTS
- mesial temporal sclerosis
- TLE
- temporal lobe epilepsy
- VBM
- voxel-based morphometry
- WM
- white matter
MTS is the most common epileptogenic lesion found in mesial TLE. It is characterized by gliosis and atrophy of the hippocampus and related limbic structures such as the mammillary body and fornix.1–3 Although most MR imaging studies focus on the limbic structures, changes in the extrahippocampal and extratemporal areas have also been observed in patients with mesial TLE.4–6 However, although the main neuropathologic substrate of hippocampal atrophy has been identified as neuron loss,7–9 the substrate of extrahippocampal and extratemporal atrophy is not fully understood. Because conventional MR imaging has limited specificity, the interpretation of GM and WM differences between patients with epilepsy and healthy controls may be problematic when subtle changes in brain structure are observed in association with neurologic and psychiatric diseases. For example, it is unclear whether the VBM changes in GM and WM observed in patients with mesial TLE are related to changes in the neuropil, neuronal size, demyelination, or dendritic or axonal arborization.
MTI is a form of tissue contrast imaging based on the exchange of longitudinal magnetization between the pool of protons bound to macromolecules (eg, myelin and cell membranes) and protons bound to water. This magnetization transfer can be measured by using specific MR imaging sequences and postprocessing tools.10,11 The magnetization transfer phenomenon is proportional to the integrity of the macromolecules that are generally MR imaging−invisible. In recent years, MTI has been used to study various neurologic disorders, particularly those such as MS that involve the WM. A number of previous studies since the early 1990s have used MTI to show cerebral tissue damage not apparent on conventional MR imaging, suggesting that MTI is sensitive to subtle changes like demyelination due to wallerian degeneration12; MS lesions,11 including demyelination and axonal loss13; and for monitoring of various CNS diseases.14 Recent studies have shown evidence that MTI can detect structural brain changes not visible with conventional volumetric MR imaging.15 In this study, to increase the specificity of the analysis, we combined conventional volumetric MR imaging and MTI protocols to evaluate extratemporal damage in patients with TLE.
Materials and Methods
Study Design
This was a prospective study comparing changes in brain volume and the macromolecular content of GM and WM between patients with mesial TLE and healthy controls. We proceeded in 3 steps: First, we compared brain volume changes (brain atrophy) between groups by using VBM. Then, we compared changes in the macromolecular content between groups by using voxel-based analysis of MTR. Finally, we investigated whether the volumetric changes obtained by VBM analyses were correlated with changes in the molecular content obtained by voxel-based analysis of MTR.
Participants
We prospectively evaluated 21 consecutive patients with mesial TLE at the outpatient clinic for refractory epilepsy between 2006 and 2008. The inclusion criteria were as follows: 1) seizure semiology consistent with mesial TLE, usually epigastric, autonomic, or psychic auras followed by behavioral arrest, progressive clouding of consciousness, oroalimentary and manual automatisms, and autonomic phenomena; 2) unilateral or bilateral anterior and mesial temporal interictal spikes; 3) video electroencephalogram monitoring with seizure onset arising exclusively from the temporal lobe; 4) MR imaging with unequivocal HA and a hyperintense signal intensity on T2-weighted sequences, with no other lesion identified; and 5) medically refractory TLE, defined as a failure to respond to at least 2 antiepileptic drugs after adequate trials. Twelve patients had right HA, 8 patients had left HA, and 1 patient had bilateral HA. Twenty-three relatives of patients with TLE with no previous neurologic disorders matched for age and sex composed the healthy controls group. The mean age at evaluation was 38.62 ± 9.02 years for mesial TLE and 37.6 ± 10.9 years for healthy controls.
Image Acquisition
All subjects were scanned with 1.5T MR imaging equipment (Magnetom Vision; Siemens, Erlangen, Germany) by using a commercially available head coil with circular polarization. The MR imaging protocol included the following: 1) a sagittal T1-weighted 3D magnetization-prepared rapid acquisition of gradient echo sequence with TR = 9.7 ms, TE = 4 ms, NEX = 1, flip angle = 12°, acquisition matrix = 256 × 256, and FOV = 25 cm, which produced 160 contiguous sections, each 1 mm thick, and a 1-mm3 isotropic voxel; 2) axial double-echo turbo spin-echo proton-attenuation T2-weighted imaging with TR = 4000 ms, TE = 22–90 ms, NEX = 1, FOV = 240 mm, and 20 sections, each 5 mm thick with a 1-mm gap; 3) a pair of fluid-attenuated inversion recovery sequences, a transverse 5-mm-thick section and a coronal 3-mm-thick section angled perpendicularly to the hippocampus, both with TR = 9000 ms, TI = 2200 ms, TE = 119 ms, FOV = 240 cm, NEX = 1, and matrix = 192 × 256; 4) a coronal turbo short tau inversion recovery sequence through the temporal lobes with thin continuous sections of 2 mm angled perpendicularly to the hippocampus with TR = 7700 ms, TI = 200 ms, TE = 60 ms, FOV = 200 cm, NEX = 2, and matrix = 154 × 256; 5) For the magnetization transfer study, we included two 3D spoiled gradient-recalled-echo sequences with TR = 34 ms, TE = 11 ms, flip angle = 30°, FOV = 256 × 192 rectangular matrix, by using 3.0-mm-thick sections in an axial plane. The first sequence did not have a magnetization transfer saturation pulse, while the second used a specific saturation pulse for solid components, with a very short relaxation time, Gaussian type, of 7.68-ms duration with 500° (effective pulse angle) and 1.5-kHz off-resonance. With these off-resonance pulses, it is possible to substantially saturate the broad resonance of the “bound pool” while hardly affecting the “free pool.”
The sequences described in items 1 and 5 were used for VBM and MTR analyses, respectively. The study was approved by our hospital ethics committee, and all subjects gave written informed consent to participate.
Postprocessing
VBM Analysis.
All of the images were converted to the Analyze format (http://imaging.mrc-cbu.cam.ac.uk/imaging/FormatAnalyze). Volume measurements were performed with optimized VBM.16 All anatomic data were processed by using the VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm) with the SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm). The toolbox used a segmentation algorithm from SPM5. The VBM5 toolbox was used for structural imaging analysis. Following the described procedure, statistical analyses were carried out with a voxel-based comparison of the WM and GM volumes between patients with TLE and healthy controls. A threshold of P < .05, corrected for multiple comparisons (FDR-corrected P values) was used for the group-difference t map to detect local changes in brain volumes.
MTR Map Construction.
The MTR map is a percentage-difference image that is calculated, voxel-by-voxel, from 1 pair of identical sequences, except for the magnetization transfer pulse, which is either added or not, by using the formula11:
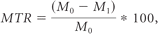 where M0 is the signal intensity of the pixel with no magnetization transfer pulse, Ms is the signal intensity of the pixel with magnetization transfer pulse, and MTR is the percentage difference map. We used the mincmath tool17 to construct the MTR map for each examination.
where M0 is the signal intensity of the pixel with no magnetization transfer pulse, Ms is the signal intensity of the pixel with magnetization transfer pulse, and MTR is the percentage difference map. We used the mincmath tool17 to construct the MTR map for each examination.
Voxel-Based MTR Analysis.
The voxel-based analysis was repeated for the magnetization transfer parameter maps. The MTR maps were spatially normalized in SPM5 to the same stereotactic space by using the methodology of Audoin et al.18 The transformation parameters were then applied to the MTR maps, and the images were smoothed with a 6-mm kernel. Two-sample t tests were carried out for the voxelwise comparison of the WM and GM volumes between mesial TLE and healthy control groups. In both VBM and MTR analyses, we did not separate patients with lesions in the left or right hippocampus.
Statistical Analysis.
We used 1-tailed tests for independent samples. Our hypothesis was that the mesial TLE group would have brain volumes and MTR measurements lower than those in the healthy control group in the VBM and voxel-based MTR analyses. P < .05 was considered statistically significant. To evaluate the correlation between VBM and voxel-based MTR, we performed the Pearson correlation test. Absolute Z values of the difference among brain volumes and MTR measurements for each brain region were calculated during VBM and voxel-based MTR analyses.
Results
Clinical Characteristics of Patients with Mesial TLE
Eleven patients were women. The mean age at seizure onset was 12.9 ± 9.7 years, the age at evaluation was 38.4 ± 9.3 years, and the mean epilepsy duration was 25.5 ± 12.3 years. Ten of 21 (48%) patients had a previous history of an initial precipitating injury (febrile seizures [n = 4], meningitis [n = 3], afebrile status epilepticus [n = 2], and traumatic brain injury [n = 1]). No patient had abnormalities on neurologic exami-nation other than material-specific memory deficits revealed during neuropsychological examination. Eight patients, despite fulfilling the inclusion criteria for mesial TLE, were not referred for surgery due to refusal (n = 4), psychosis (n = 2), or high risk of permanent memory deficits indicated by World Anti-Doping Agency test (n = 2). Thirteen patients underwent anterior temporal lobectomy. In all patients, histopathologic examination revealed no lesion other than hippocampal sclerosis.
Results of Volumetric Analysis by Using VBM
On-line Table 1 shows the results of VBM analyses of GM and WM volumes for the mesial TLE and healthy control groups. Volumetric analysis by using VBM identified a volume loss in 20 GM regions and 19 WM regions of the TLE group compared with the healthy control group. In the GM, the areas with significant volume reduction were predominantly the frontal lobe, temporal lobe, and basal ganglia (On-line Table 1). As expected, significant changes were found in limbic structures such as the amygdala (P = .001), parahippocampal gyrus (P = .001), and the limbic portion of the anterior cingulate gyrus (P = .018). However, changes were not restricted to those regions but were found with a widespread pattern. In the WM, the volume loss occurred predominantly in the corpus callosum (P = .001) and the frontal and temporal lobes. Additionally, a significant loss of volume was found in the postcentral gyrus (P = .001) and the middle occipital gyrus (P = .018) (On-line Table 1).
Results of Voxel-Based MTR Analysis
On-line Table 2 shows the voxel-based MTR analysis of the mesial TLE and healthy control groups. Voxel-based MTR analysis identified MTR reduction in 20 GM regions and 17 WM regions in the TLE group compared with the healthy control group. There were significant reductions in the frontal and temporal lobes, the caudate head (P < .001), caudate body (P < .001), putamen (P = .002), mammillary body (P = .001), frontal lobe cortex (P = .002), and temporal lobe cortex (P = .003). The MTR changes within the WM occurred in the corpus callosum (P < .001), midbrain (P < .001), insula (P = .002), limbic lobe (P = .001), parahippocampal gyrus (P = .001), anterior cingulate (P = .004), temporal lobe, parietal lobe, precuneus (P < .001, Z = 3.80), frontal lobe, and occipital lobe.
Correlation between GM and WM VBM and Voxel-Based MTR
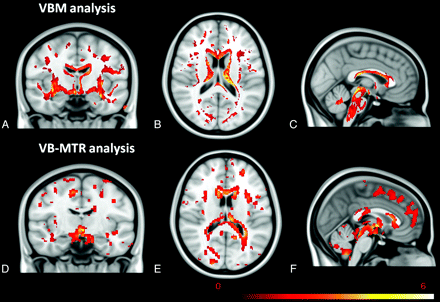
There was no correlation between VBM and voxel-based MTR in the GM (r = 0.662, P = .105); however, a strong and positive correlation was observed between VBM and voxel-based-MTR in the WM (r = 0.815, P = 0.001). Fig 1 shows t maps from VBM and voxel-based MTR analysis. It is possible to observe common regions in both analyses.
The first row (A−C) shows the VBM results, and the second row (D−F) shows the VB-MTR results in WM, both registered with Montreal Neurological Institute 152 template. Note common regions in both analyses and additional regions in voxel-based MTR analysis with reduced MTR but without atrophy.
Discussion
The goal of our study was to evaluate tissue changes associated with extrahippocampal and extratemporal volume loss in patients with mesial TLE. In combination with volumetric analysis by using VBM, we performed MTR analysis to quantify the protons bound to macromolecules in brain regions of patients with mesial TLE compared with healthy controls. In addition to the expected MTR decrease in the temporal lobes, we found a significant decrease in the WM and GM of the whole brain. In the WM, the MTR decrease was correlated to volume loss detected by VBM, indicating that brain atrophy may explain part of the MTR decrease. However, we also found areas where the MTR decrease was not associated with volume loss, suggesting an additional pathophysiologic process other than neuronal loss and atrophy underlying the MTR changes. Thus, our data support the notion that MTR measurements can detect structural brain changes not visible with volumetric MR imaging and support the view that the combined use of these 2 MR imaging modalities may provide useful insights for elucidating structural brain abnormalities in mesial TLE.
MTI provides an indication of the quantity of bound protons in a given tissue, which is reduced in many pathologic processes, particularly in those associated with demyelination and axonal damage.19 Changes in MTR have been reported both in experimental and clinical studies. Experimental studies by using a feline model of a controlled optic nerve lesion followed by serial MTI have shown that this method is more sensitive for early detection of wallerian degeneration of the anatomic pathways than conventional MR imaging.12 Clinical studies have reported that MTR measurements are minimally affected in cerebral edema,11 and major reductions are observed in more severe processes related to several CNS diseases.20 In recent years, MTR has been one of the MR imaging techniques most extensively used for the assessment of CNS lesions in MS,14,21 with the added advantage of being able to predict disease progression.22 Although brain atrophy may also be associated with the MTR decrease, pathologic correlations have demonstrated that the main substrate of the MTR decrease is demyelination. In addition, in normal-appearing WM, MTR decrease correlates with axonal attenuation.23 Therefore, we speculate that the MTR decrease in our cohort may be explained by a combination of neuronal and axonal damage and demyelination. However, neuropathologic studies are needed to elucidate the pathologic substrate of the MTR changes found in patients with mesial TLE.
Our data confirm previous reports showing the presence of extratemporal WM lesions in animal models24 and in humans with mesial TLE.25–29 For example, studies have shown regional differences in GM and WM between groups of patients with TLE and healthy controls that extend beyond the temporal lobe; these differences are also observed in brain tissue connected to hippocampal structures such as the cingulum, thalamus, and frontal lobe.29–35 Our data are in line with other reports showing reduced GM and WM volumes in temporolimbic and frontal areas.31,33 Abnormalities in these regions in patients with mesial TLE have been reported by others by using diffusion tensor imaging.27,36,37 The frontal damage in patients with TLE might be explained by pathologic studies that have demonstrated extensive reciprocal connections between the prefrontal cortex, the mesial temporal lobe, and the thalamus. These connections are known as the frontolimbic network,38–40 which is crucial for cognitive functions such as memory, attention, and emotional behavior.41
Some limitations of our study are related to the VBM and MTR acquisition and postprocessing. VBM is sensitive to methodologic choices in normalization, smoothing kernel, and template.42,43 In this study, we tried to minimize these limitations by using an appropriate template. MTR is sensitive to instrumental factors and MR imaging hardware imperfections such as transmitter coil nonuniformity as well as inaccuracy and instability in the magnetization transfer pulse power.19 To minimize these problems, examinations of patients and healthy controls were performed during the same time period. Another limitation of our study was the small sample size. However, our study found several areas of MTR decrease in patients with TLE compared with healthy controls. When we perform multiple comparisons, type I error might be a problem. To control for type I error, we used the FDR. An increase in the healthy control sample size might show additional areas with MTR decrease, but it is unlikely that we would see changes in the areas that already had a significant MTR decrease.
We have found a strong positive correlation between VBM and voxel-based MTR in the WM (r = 0.815), which might suggest that not only does tissue loss occur but also demyelination and/or axonal loss, because MTI assesses the quality of the remaining tissue after volume reduction. On the other hand, we did not a find correlation between VBM and voxel-based MTR in the GM (r = 0.662, P = 1.05), which might suggest other concurrent phenomena like extracellular space, with a different influence on volume and tissue attenuation versus membrane and macromolecular loss.
Conclusions
We found WM and GM changes beyond the temporal lobe in patients with TLE. These abnormalities were more intense in regions connected with the temporal lobe, such as the frontal lobe, suggesting that mesial TLE is a network disease that causes widespread lesions in both GM and WM; these lesions may possibly involve demyelination. Moreover, our data support the notion that MTR measurements can detect structural brain changes not visible with volumetric MR imaging and support the view that the combined use of these 2 MR imaging modalities may provide useful insights into the structural brain abnormalities in mesial TLE.
Footnotes
-
This work was partially supported by the Brazilian agencies FAPESP and CNPq. Paula R.B. Diniz was supported by FAPESP. A. Carlos Santos, Joao P. Leite, and Tonicarlo R. Velasco were supported by CNPq.
-
Disclosures: Paula R.B. Diniz: Research Support (including provision of equipment or materials): FAPESP, Research Support (including provision of equipment or materials): CINAPCE Project, FAPESP, Details: governmental agency for supporting research. Joao P. Leite: Research Support (including provision of equipment or materials): CINAPCE, Details: a governmental funding agency. FAPESP Project 05/56447–7.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received December 24, 2010.
- Accepted after revision February 22, 2011.
- © 2011 by American Journal of Neuroradiology