Abstract
BACKGROUND AND PURPOSE: We have developed PC HYPRFlow, a comprehensive MRA technique that includes a whole-brain CE dynamic series followed by PC velocity-encoding, yielding a time series of high-resolution morphologic angiograms with associated velocity information. In this study, we present velocity data acquired by using the PC component of PC HYPRFlow (PC-VIPR).
MATERIALS AND METHODS: Ten healthy volunteers (6 women, 4 men) were scanned by using PC HYPRFlow and 2D-PC imaging, immediately followed by velocity measurements by using TCD. Velocity measurements were made in the M1 segments of the MCAs from the PC-VIPR, 2D-PC, and TCD examinations.
RESULTS: PC-VIPR showed approximately 30% lower mean velocity compared with TCD, consistent with other comparisons of TCD with PC-MRA. The correlation with TCD was r = 0.793, and the correlation of PC-VIPR with 2D-PC was r = 0.723.
CONCLUSIONS: PC-VIPR is a technique capable of acquiring high-resolution MRA of diagnostic quality with velocity data comparable with TCD and 2D-PC. The combination of velocity information and fast high-resolution whole-brain morphologic angiograms makes PC HYPRFlow an attractive alternative to current MRA methods.
Abbreviations
- CE
- contrast-enhanced
- CE-VIPR
- contrast-enhanced vastly undersampled isotropic projection reconstruction
- 2D-PC
- 2D phase-contrast
- HYPR-LR
- highly constrained local projection reconstruction
- MCA
- middle cerebral artery
- MRA
- MR angiography
- PC
- phase-contrast
- PC HYPRFlow
- time-resolved MRA using highly constrained projection reconstruction and PC-VIPR data for the reconstruction convolution
- PC-MRA
- phase-contrast MR angiography
- PC-VIPR
- phase-contrast vastly undersampled isotropic projection reconstruction
- SNR
- signal intensity–to-noise ratio
- TCD
- transcranial Doppler sonography
- VENC
- velocity encoding
- WSS
- wall shear stress
The acquisition of intracranial velocity measurements and velocity derivatives (WSS) is clinically useful for the evaluation of neurovascular disorders such as vasospasm, stenoses, and aneurysms, but measurement of intracranial velocity has proved challenging. TCD has been used clinically for decades and allows the acquisition of velocity measurements in the MCAs and several other vessels through the temporal window. However, the sonic properties of the cranial vault prevent measurement of velocity in many intracranial arteries by TCD. 2D-PC MR imaging has been used to obtain hemodynamic data for the past 20 years but has limited coverage and exhibits partial volume effects.1 3D and 4D methods have higher SNR, fewer partial volume effects, and improved spatial resolution but have an increased scanning time.2 Previous implementation of whole-brain cardiac-gated PC Cartesian 4D MR imaging has resulted in scanning times too long to be clinically useful.3,4 Advances in PC Cartesian acquisition have reduced scanning times and increased resolution to some degree. Investigators have used view-sharing,5 spatial harmonics,6 and parallel imaging7 to reduce scanning time. With these methods, in-plane spatial resolutions on the order of 0.6 × 1 mm with scanning times in the 8- to 12-minute range can be achieved.8.
However, obtaining high-resolution whole-brain angiograms with velocity information within clinically useful imaging times has been challenging. Recently, we implemented radial imaging techniques that are particularly well-suited for neurovascular MRA. Because MRA is sparse, consisting of few changing nonzero elements, accelerated acquisitions can often be performed with tolerable artifacts. For example, azimuthally undersampled radial acquisitions can be used to acquire images in much shorter scanning times than Cartesian acquisitions of a similar resolution with acceptable SNR and image quality,9 due to the relatively benign nature of streaklike artifacts caused by the undersampling.10,11
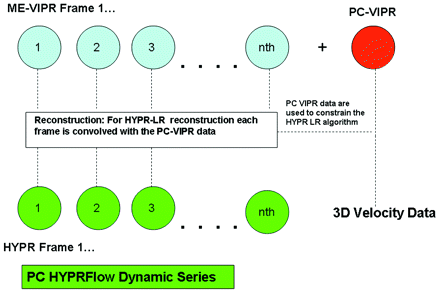
We have combined radial undersampling with a novel constrained image reconstruction technique to create PC HYPRFlow,9,12 a comprehensive MRA technique. Figure 1 shows a flow chart of how images are acquired and reconstructed by using PC HYPRFlow. A series of low-resolution 3D-radial CE source images are acquired (CE-VIPR), followed by a high-resolution velocity-encoded PC acquisition (PC-VIPR13). The low-resolution source images are then convolved with the high-resolution PC velocity acquisition by using a technique called HYPR-LR14 to yield a time series of high-resolution morphologic angiograms with associated velocity information. With current protocols, PC HYPRFlow provides whole-brain angiograms with excellent spatial resolution (0.68 × 0.68 × 0.68 mm3) with scanning times of 5–6 minutes.9
Flow chart of PC HYPRFlow. First a series of low-resolution CE source images is acquired, followed by a high-resolution velocity-encoded PC acquisition. Then the low-resolution source images are convolved with the high-resolution PC image by using HYPR-LR, resulting in a time series of high-resolution HYPR images.
Because PC HYPRFlow is a highly accelerated technique, it may be susceptible to errors. To ensure diagnostic confidence, we have conducted several studies to validate image quality and velocity measurements from PC HYPRFlow.9,15–17 We previously reported that PC HYPRFlow provides images of diagnostic quality in medium- and large-sized intracranial vessels.9 In this study, we investigated velocity information acquired by using the PC component of PC HYPRFlow. We validated velocity measurements acquired by using PC-VIPR in the MCAs of healthy volunteers by comparing them with velocity measurements from 2 reference standards, TCD and Cartesian 2D-PC-MRA. The purpose of this comparison was to define the relationship of PC HYPRFlow velocity measurements to other established modalities.
Materials and Methods
Volunteer studies were performed in compliance with Health Insurance Portability and Accountability Act regulations and by using a protocol approved by the local institutional review board. Ten healthy volunteers ranging from 19 to 58 years of age were imaged (6 women, 4 men) with a clinical 3T MR imaging system (MR HD 750; GE Healthcare, Milwaukee, Wisconsin) with a 8-channel head coil (Excite HD Brain Coil, GE Healthcare). Before contrast injection, a fast 2D-PC scan of each MCA was obtained to estimate the velocity to determine optimum VENC to prevent aliasing, followed by a PC HYPRFlow acquisition. Two fast low-resolution scans (2 × 2 × 2 mm3) were used in the time-resolved multiecho 3D radial acquisition (CE-VIPR).18 Contrast was injected during the second scan. Subsequently, velocity encoding was performed by using a high-resolution dual-echo 3D-radial PC acquisition (PC-VIPR). The PC-VIPR data were used as a composite image (angiographic constraint) for HYPR-LR reconstruction and for hemodynamic evaluation.14 Immediately following the MR imaging examination, the volunteers underwent TCD scanning.
MR Imaging Protocol
Imaging parameters for CE-VIPR were the following: FOV = 26 × 26 × 26 cm2, TR/TE = 3.0/0.4 ms, bandwidth = 125 kHz. For each projection, there were 64 points from the center to the edge of the k-space, with a frame update time of 0.75 seconds. Scanning parameters for postcontrast PC-VIPR were the following: FOV = 22 × 22 × 22 cm2, TR/TE = 12.5/4.8 ms, VENC = 80–150 cm/s, bandwidth = 83.3 kHz, readout matrix = 320 points per projection, spatial resolution for the composite image = 0.68 × 0.68 × 0.68 mm3. Seven thousand projections were acquired within 5 minutes. Gadobenate dimeglumine (MultiHance; Bracco Diagnostics, Princeton, New Jersey) was injected at 3 mL/s, and the contrast dose was 0.1 mm/kg followed by a 20-mL saline flush. The MR imaging acquisitions were cardiac gated by using chest leads (Table).
Imaging parameters
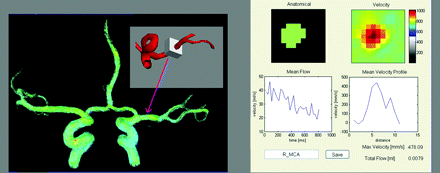
A series of 3D time-resolved velocity images were reconstructed from the PC-VIPR data. A MatLab inhouse developed software filter with 50 ms at a low spatial frequency and 130 ms at a high spatial frequency was applied to improve the SNR. A vascular mask was generated by applying a signal-intensity threshold on the complex difference image. Subsequently, a region of interest, including approximately 100 voxels, was chosen in the M1 segment of the MCA as shown in Fig 2. Velocity measurements were made by using an in-house flow tool.19 Mean velocity was averaged within the region of interest for each time frame. Mean velocity through the whole cardiac cycle was calculated for each volunteer. The maximum velocity over the cardiac cycle was designated as the peak systolic velocity, and the minimum velocity was designated as the minimum diastolic velocity. This process was repeated for data from the 2D-PC acquisition.
A 100-voxel box is selected in each MCA, and velocity measurements are automatically acquired by using an in-house flow tool.
TCD Protocol
TCD imaging was performed in the MCAs bilaterally by using an Acuson-Sequoia sonography scanner (Siemens, Malvern, Pennsylvania) and a 4V1 MHz vector transducer. Depths for MCA interrogation were retrieved from the subjects' MRAs and given to the sonographer. The sonographer would attempt to replicate the depths when obtaining spectral Doppler signals. Color and spectral Doppler images were obtained via the transtemporal window. Once spectral Doppler images were obtained, mean, peak systolic, and end diastolic velocities were recorded. Three velocity measurements were obtained in each MCA.
Statistical Methods
Correlations were acquired between PC-VIPR and TCD and PC-VIPR and 2D-PC. Bland-Altman plots were generated comparing PC-VIPR and TCD and PC-VIPR and 2D-PC, and the bias and limits of agreement were calculated for each Bland-Altman plot. Statistics were calculated by using Excel 2007 (Microsoft, Bothell, Washington). The Pearson r was used for correlation analysis, and a P value < .05 for the correlation coefficient was considered statistically significant.
Results
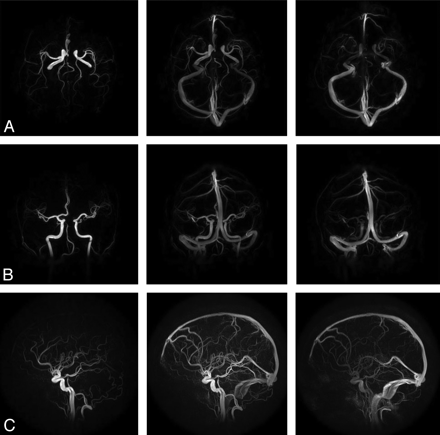
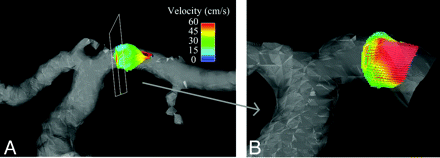
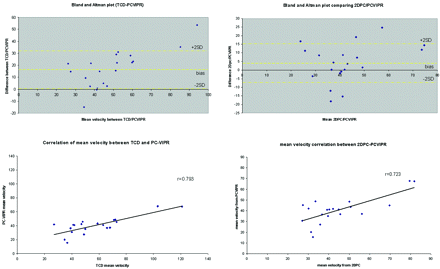
Figure 3A–C shows axial, coronal, and sagittal whole-brain images acquired during the first pass of the contrast bolus by using PC HYPRFlow in the early arterial, mixed, and venous phases. Figure 4A, shows a velocity vector plot of the carotid terminus and the MCA and Figure 4B is a cross-section of the MCA showing velocity vectors. Both plots show the parabolic distribution of velocities. The mean velocity (±SD) measured by sonography in the left and right MCAs of all volunteers (n = 20) was 57.54 ± 29.30 cm/s, peak systolic velocity was 85.21 ± 30.78 cm/s, and diastolic velocity was 41.42 ± 17.47 cm/s. Mean velocity measured by 2D-PC was 44.69 ± 16.09 cm/s, peak systolic was 64.59 ± 19.91 cm/s, peak diastolic was 29.79 ± 10.98 cm/s, and mean velocity measured by PC-VIPR was 43.17 ± 10.49 cm/s. Peak systolic was 58.39 ± 10.86 cm/s, and peak diastolic was 31.32 ± 12.00 cm/s. Bland-Altman analysis of PC-VIPR compared with TCD showed 17 of 20 points within 2 SDs, with a bias of 16.9 and 2 SD limits of agreement of 1.6 and 32, which is consistent with the values found in the literature showing that mean velocities acquired with PC-MRA are approximately 30% lower than those acquired by using TCD.20–24 Bland-Altman analysis of PC-VIPR compared with 2D-PC showed 14 of 20 points within 2 SDs, with a bias of 4.05 and 2 SD limits of agreement of −7.15 and 15.2. PC-VIPR and TCD were well correlated, with r = 0.793. PC-VIPR and 2D-PC showed a correlation of r = 0.723. P values of both correlations were <.001. Figure 5 shows the Bland-Altman and correlation plots between PC-VIPR and TCD (top) and PC-VIPR and 2D-PC (bottom).
Representative images from a 60-frame time-series of the whole brain acquired by using PC HYPRFlow showing the early arterial, mixed, and venous phases: axial (A), coronal (B), and sagittal (C).
A, Segmentation of the carotid terminus showing velocity vectors in the MCA. B, Enlargement of a cross-section of the M1 segment of the MCA showing parabolic distribution of velocities in the MCA.
Top left: Bland-Altman plot of PC-VIPR compared with TCD. Top right: Bland-Altman plot of PC-VIPR compared with 2D-PC. Bottom left: Correlation plot of PC-VIPR with TCD. Bottom right: Correlation plot of PC-VIPR with 2D-PC.
Discussion
Velocity measurements are clinically useful both alone and in conjunction with vessel morphology and derivative measurements in identifying the extent and clinical impact of stenoses.8 The time-series, velocity measurements, and velocity derivatives acquired by using PC HYPRFlow demonstrate that this approach is an attractive alternative to other time-resolved and PC-MRA methods. We have previously shown that time-resolved CE PC HYPRFlow provides whole-brain images of diagnostic quality for the assessment of vascular structures as small as 2.0 mm in diameter.9 In this study, we demonstrated that the PC-VIPR component of PC HYPRFlow is capable of velocity measurements consistent with 2 reference standards, product 2D-PC-MRA and TCD.
TCD is commonly used for velocity measurements, but the thickness of the skull makes it challenging to obtain measurements from many intracranial blood vessels. While the temporal bone window allows the proximal MCA and parts of the internal carotid artery to be visualized, other important segments are difficult or impossible to measure by using TCD. In this study, we showed that PC-VIPR provides velocity measurements from the MCA that are consistent with other PC-MRA/TCD correlations in the literature.20–24 In general, due to temporal averaging, velocity measurements by using PC-MRA are 30% less than those of TCD because TCD essentially has real-time temporal resolution, making it easier to sample the maximum-velocity near-peak systole. PC-MRA accuracy is influenced by several factors that have the potential to report lower maximum velocities. First, PC-MRA is a gated technique. If the peak velocity position and amplitude are shifted from heartbeat to heartbeat, PC-MRA will capture an average velocity over multiple heartbeats. Also, PC-MRA assumes a single velocity/voxel model. Thus, disturbed flow and resolution effects limit the detection of peak velocities. Finally, the temporal resolution of PC-MRA is substantially lower than that in sonography, causing PC-MR imaging to temporally blur and lower measured peak velocities.23,24 Nonetheless, the velocities were well-correlated and can easily be used to compare baseline velocities from those seen in pathologic conditions. The values acquired by using a whole-brain PC-VIPR acquisition were also similar to those acquired by using product 2D-PC-MRA in a much smaller FOV. PC-VIPR is capable of acquiring velocity measurements from the whole brain without the limitations of sonography, while also providing whole-brain 4D angiograms showing morphology and a time-series.
Emerging data indicate a link between abnormal WSS and the development and progression of both atherosclerotic plaques25,26 and saccular and fusiform cerebral aneurysms.8,27 Previous efforts to calculate WSS with PC-MRA have had limited success because of lack of sufficient spatial resolution within clinically useful scanning times,28 boundary-zone localization, and aliasing, especially in areas of stenosis. PC-VIPR acquires whole-brain angiograms with high spatial resolution (0.68 × 0.68 × 0.68 mm3), which provides 8–12 pixels within the MCA. Using automated spline interpolation,28,29 we were able to visualize the boundary zone and acquire automated WSS calculations by using velocity data from PC-VIPR. We have also introduced a 5-point velocity-encoding tool that is capable of decreasing velocity aliasing.30 This helps acquire velocity measurements in areas of high velocity, such as stenoses.
Limitations
While PC HYPRFlow has high spatial resolution compared with techniques in current clinical use, the spatial resolution is still relatively low compared with that typically used to calculate WSS in computational fluid dynamics31 and digital subtraction angiography in the acquisition of morphologic angiograms. At this time, this limitation restricts the vessels that can be imaged with diagnostic quality with PC HYPRFlow to medium- and large-sized vessels. However, with the advent of 32-channel head coils, higher spatial resolution will be achievable, allowing smaller vessels to be imaged and velocity and WSS to be calculated in smaller vessels as well.32 Similarly, these changes can also improve temporal resolution, allowing peak systolic velocity to be better detected. Patient motion is an issue, but we have implemented image registration to correct this problem.
Conclusions
PC HYPRFlow is a comprehensive technique that acquires both morphologic images of diagnostic quality and velocity data in 5–6 minutes. The PC-VIPR component of PC HYPRFlow provides velocity measurements consistent with 2D-PC and TCD in medium and large intracranial vessels.
Acknowledgments
We thank the following individuals for their important contributions to this project: Sara Baker, MEd, RT, and Carol Mitchell, PhD, for supervision and performance of the TCD studies; Sara Pladziewicz, RT, and Kelli Hellenbrand, RT, for performance of the PC HYPRFlow examinations; and Kari Pulfer, RT, for subject recruitment.
Footnotes
-
This work was supported by National Institutes of Health grant R21 EB009441 to Patrick Turski.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received March 15, 2010.
- Accepted after revision June 1, 2010.
- Copyright © American Society of Neuroradiology