Abstract
BACKGROUND AND PURPOSE: Thromboembolic events related to CAS continue to be the main limitation to the widespread use of this technique as a first-line treatment for carotid occlusive disease. Our aim was to evaluate thromboembolism during CAS using DWI for catheterization techniques of the carotid artery.
MATERIALS AND METHODS: Thirty-two consecutive patients with symptomatic carotid stenosis underwent CAS involving 1 of 2 carotid artery catheterization techniques: One used a 7F or 8F catheter (group 1, n = 16) and the other used a coaxial system in which a 7F or 8F catheter was used in conjunction with a 4F or 5F catheter (group 2, n = 16). DWI was performed before and after CAS. Clinical variables, the number and location of NES on DWI after CAS, were compared between the 2 groups.
RESULTS: NES on DWI occurred in 53% of all patients. The incidence of NES was significantly higher in patients 65 years of age and older versus those younger than 65 years of age (P = .013). All NESs were asymptomatic, and their rate of occurrence did not differ significantly between groups 1 and 2. The incidence of NES in the other territories that were outside that of the treated carotid artery (P = .004) and the incidence of multiple NESs (P = .04) were significantly higher in group 1.
CONCLUSIONS: NES in the other territories mainly arises from the atherosclerotic aortic arch and arch vessels during the manipulation of endoluminal devices. The carotid artery catheterization technique using the coaxial system with a 7F or 8F catheter in conjunction with a 4F or 5F catheter reduced the incidence of NES in the other territories.
Abbreviations
- ACA
- anterior cerebral artery
- ACT
- activated clotting time
- CAS
- carotid artery stenting
- CCA
- common carotid artery
- CEA
- carotid endarterectomy
- DWI
- diffusion-weighted imaging
- ICA
- internal carotid artery
- NES
- new embolic signal
Although CAS is increasingly used to treat extracranial carotid stenosis due to its less invasive nature, it has not been shown to be safer and more effective than CEA.1–4 Thromboembolic events related to CAS continue to be the main limitation in the widespread use of this technique as a first-line treatment for extracranial carotid occlusive disease. Cerebral protection devices have, therefore, been used to limit cerebral embolism in patients undergoing CAS.
New ischemic lesions after invasive cerebrovascular procedures are often seen on DWI. Although they may not be clinically overt, these lesions represent the overall thromboembolic risk of these procedures. DWI used to evaluate procedure-related embolic events in patients undergoing CAS has shown that NES can occur in the vascular territory of the treated artery as well as in the other territories that are outside the territory of the treated artery.5–12 Cerebral protection devices have been shown to reduce the number of NES in the territory of the treated artery but have no effect on lesions in the other territories.10 These results indicate that the main determinant of thromboembolic events in CAS may be the individual patient's underlying diffuse atheromatosis or embolization resulting mainly from manipulation of the catheter, wire, or sheath in the aortic arch and arch vessels. Great care is, therefore, needed, especially during the initial phase of the procedure before filter placement and the CAS procedure itself. In this phase, several catheterization techniques are used for carotid artery access, depending on the arch extension and tortuosity of arch vessels, with different combinations of catheters and guidewires or different exchange maneuvers.13,14
Using DWI before and after CAS, we evaluated thromboembolism during CAS according to the carotid artery catheterization technique.
Materials and Methods
Patients
Of the 43 patients who underwent CAS from January 2007 to December 2008, we chose 32 consecutive symptomatic patients with >50% atherosclerotic stenosis. Patients were excluded for the following reasons: 1) They were asymptomatic, 2) did not undergo pre- or postprocedural DWI, 3) had new ischemic symptoms between preprocedural DWI and the procedure, 4) underwent CAS in hyperacute thrombolysis, or 5) had an exchange maneuver in introducing a guiding catheter into the targeted CCA due to tortuosity or elongation of the aortic arch and acute angulation of the brachiocephalic artery or left CCA from the aortic arch. All patients gave written informed consent for the procedure. Patients were clinically evaluated by independent neurologists and neurosurgeons before and immediately after the procedure, at discharge, and at regular follow-up intervals of 1–3 months. Our institutional review board approved this retrospective study.
MR Imaging
Our routine MR imaging protocol includes preprocedural and postprocedural evaluations in patients undergoing CAS for atherosclerotic stenosis. At admission, patients underwent MR imaging with a preprocedural protocol and were scheduled for diagnostic cerebral angiography and subsequent CAS. MR imaging was performed by using a 1.5T whole-body-system imager (Intera; Philips Healthcare, Best, the Netherlands). Our preprocedural MR imaging protocol included DWI, a fluid-attenuated inversion recovery sequence, a gradient-echo sequence, 3D time-of-flight MR angiography, and contrast-enhanced MR angiography or MR perfusion imaging. Our postprocedural MR imaging protocol included DWI within 3 days after the procedure. DWI was performed by using a single-shot echo-planar imaging sequence with sensitivity encoding and a parallel-imaging scheme. All images were reviewed by 2 experienced neuroradiologists (H.J.K and P.S.Y.).
New diffusion-prolonged foci on postprocedural DWI not seen on preprocedural DWI were considered procedure-related embolic signals. The embolic signals were described by their number (single and multiple) and location (the territory of the treated artery and the other territories). We defined the “territory of the treated artery” as the area supplied by the angiographically treated carotid artery—that is, if the contralateral ACA territory was predominantly demonstrated on the angiogram of treated carotid artery, the ACA territory was considered as the territory of treated artery. Areas that were outside of this distribution were defined as the “other territories.”
CAS Procedure
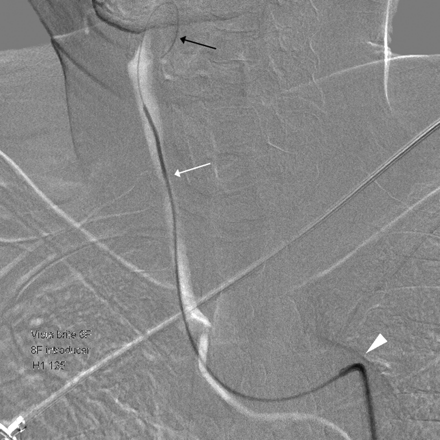
This was not a prospectively designed study; rather, it was based on collected data in the daily practical field. CAS was performed by direct selection of the brachiocephalic artery and left CCA by using a 7F or 8F guiding catheter (Envoy; Cordis, Miami Lakes, Florida), primarily during 2007. Using this method, we found a >60% incidence of NES on postprocedural DWI. Because the literature has revealed that a smaller catheter with a properly angulated tip (ie, Headhunter diagnostic catheter; Cook, Bloomington, Indiana) is less traumatic,15–18 we changed the method of introducing the guiding catheter to reduce the incidence of NES on postprocedural DWI. Using this method, we introduced a 90-cm-long 7F or 8F guiding catheter into the femoral sheath with a 120-cm-long 4F or 5F diagnostic catheter coaxially and crossed the aortic arch, selected the arch vessels, and positioned it at the targeted region of the CCA with a diagnostic catheter ahead of the guiding catheter (Fig 1). We removed the diagnostic catheter after appropriate positioning of the guiding catheter proximal to the stenotic lesion. This maneuver is also a commonly used carotid artery catheterization technique in CAS.13,14 This change resulted in a meaningful reduction in NES on DWI after CAS. This study, therefore, includes 2 nonrandomized groups of patients that differed according to the carotid artery catheterization technique: One had a 7F or 8F catheter manipulated over a 0.035-inch guidewire (group 1) and the other had a coaxial system in which a 7F or 8F catheter was used in conjunction with a 4F or 5F catheter (group 2).
Carotid artery catheterization technique by using a coaxial system. Roadmap image shows a 0.035-inch guidewire inserted into the facial artery (black arrow), following a 5F headhunter catheter in the right CCA (white arrow) and an 8F guiding catheter crossing the aortic arch (arrowhead).
Other procedural steps were the same in both groups. All patients were premedicated with daily doses of 100-mg aspirin and 75-mg clopidogrel for at least 3 days before the procedure. Following the procedure, patients were continued on 100-mg/day aspirin indefinitely and 75-mg/day clopidogrel for at least 3 months. Therapeutic procedures were performed during a second angiographic session. The patients were fully awake during the procedures, and electrocardiography, arterial oxygen saturation, and blood pressure parameters were appropriately monitored. Percutaneous access was obtained via the right femoral artery, and a 7F-9F sheath was inserted. Baseline ACT was obtained before the procedure. The patients received a bolus injection of 3000- to 5000-IU heparin just before the start of the therapeutic procedure, with a boost of 1000-IU heparin administered every hour to provide an ACT >250 seconds or twice the baseline ACT during the procedure. A 7F-8F guiding catheter was positioned proximal to the stenotic lesion in the CCA by 1 of 2 carotid artery catheterization techniques. The length and stenotic rate of the stenotic segment were calculated manually, as well as automatically, on the basis of digital subtraction angiography according to the North American Symptomatic Carotid Endarterectomy Trial criteria.19
In all patients, the stenosis was initially crossed by a 0.014-inch guidewire, and in some patients, a filter-type protection device was deployed in the cervical portion of the ICA. The cost of this protection device is not covered by health insurance in our country and must be paid for by the patient. We inform all patients of the existence, effect, and cost of the protection device before CAS and use it only in patients who agree to these conditions. If the stenotic segment was <2 mm in diameter, it was predilated with a 3-mm-diameter angioplasty balloon. A self-expandable stent delivery catheter was then advanced over the immobilized guidewire or filterwire. After stent deployment, postdilation was performed by using a 5- or 6-mm-diameter angioplasty balloon if residual stenosis was >30%. The protection device was removed. Angiograms of the carotid bifurcation and the intracranial circulation were obtained to demonstrate the reconstruction of the carotid lumen and to exclude macroembolic complications.
Statistical Analysis
All data were analyzed on an intention-to-treat basis with the Statistical Package for the Social Sciences, Version 12.0 (SPSS, Chicago, Illinois). We used the Mann-Whitney U test to compare continuous variables and the χ2 test or Fisher exact test to compare categoric variables between groups. The between-group differences in the number and location of NES on postprocedural DWI were compared by using the Fisher exact test. P < .05 was considered statistically significant.
Results
The clinical characteristics of the patients in the 2 groups are summarized in Table 1. There were 16 patients in each group: 11 men and 5 women (mean age, 66 years; range, 48–81 years) in group 1; and 10 men and 6 women (mean age, 64 years; range, 51–73 years) in group 2. Of these 32 patients, 23 presented with stroke and 9, with transient ischemic attack. There were no significant between-group differences in sex, age, and vascular risk factor profiles (Table 1). The mean time interval between symptom onset and diagnostic cerebral angiography was 14.5 days in group 1 and 18.3 days in group 2; the mean time interval between symptom onset and CAS was 17.2 and 23.7 days, respectively; the mean time interval between diagnostic cerebral angiography and CAS was 2.7 and 5.4 days, respectively; the mean time interval between preprocedural DWI and CAS was 13.3 and 16.3 days, respectively; and the mean time interval between CAS and postprocedural DWI was 1.6 and 1.1 days, respectively. The use of protection devices was significantly higher in the group 1 versus group 2 (Table 1).
Clinical and procedural variables in the 2 patient groups
The incidence of NES was significantly higher in patients 65 years of age and older (range, 65–81 years) than in patients younger than 65 years of age (range, 48–64 years) (P = .013).
The number and location of NES after CAS in the 2 groups are shown in Table 2. The rate of occurrence of NES did not differ significantly between these 2 groups (P = .72), but the incidence of multiple NES was significantly higher in group 1 (P = .04) as was the incidence of NES in the other territories (P = .004) (Fig 2). In both groups, there were no differences in the rate of occurrence of NES relative to the use of protection devices. In patients with NES, there were no correlated clinical variables between the 2 groups (Table 3).
Numbers and locations of NES on DWI after CAS in the 2 patient groups
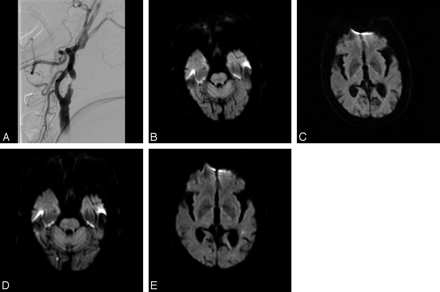
An 81-year-old man presented with transient right arm and leg weakness. A, Digital subtraction angiography shows severe stenosis of the left proximal ICA. CAS of this lesion was performed by the carotid artery catheterization technique with a 7F or 8F catheter manipulated over a 0.035-inch guidewire. B and C, DWI obtained at 1 day before CAS shows no abnormal signal intensity. D and E, DWI obtained at 1 day after CAS shows 2 small NES in the right occipital lobe and left temporal lobe.
Clinical and procedural variables in patients with NES
Postprocedural neurologic examinations showed no changes in the clinical conditions of all patients.
Discussion
We assessed whether patients undergoing CAS with 2 different carotid artery catheterization techniques have differences in thromboembolic events. We found that the carotid artery catheterized by using a coaxial system in which a 7F or 8F catheter was used in conjunction with a 4F or 5F catheter resulted in a reduced rate of thromboembolic events in the other territories that are outside the territory of the treated artery during manipulation in the aortic arch and arch vessels.
NES on DWI after CAS has appeared in the other territories as well as the territory of the treated artery.5–12 NES in the other territories may be due to emboli from the treated lesion reaching the contralateral hemisphere through the intracranial compensation supply. However, the use of a protection device did not reduce the incidence of NES in the other territories,10 and NES in the other territories has not been reported after CEA.8 These findings indicate that maneuvers in the aortic arch and arch vessels during CAS play an important role in the occurrence of NES in the other territories. Our findings also indicate that the use of less traumatic navigation methods and endoluminal devices reduces the occurrence of NES in the other territories.
The carotid artery catheterization technique with a coaxial system in which a 7F or 8F catheter was used in conjunction with a 4F or 5F catheter is commonly used in patients in whom difficulties in positioning the guiding catheter are expected due to tortuous or elongated aortas or acute angulation of the arch vessels.13,14,20 This method simplifies the selection of the arch vessels and makes it easier to navigate the guiding catheter by providing more stable support. We aimed to evaluate whether these advantages affect the rate of thromboembolic events originating from the aorta or arteries proximal to the treated lesion. These thromboembolic events were not overcome by protection devices and remain a problem in patients undergoing CAS. We found that, overall, 53% of patients had new DWI lesions, similar to the rates of 22%–54% reported previously.5–12 The incidence of NES was higher in the group 1 carotid artery catheterization technique using a 7F or 8F catheter manipulated over a 0.035-inch guidewire (62%) than in group 2 using the carotid artery catheterization technique with a coaxial system in which a 7F or 8 F catheter was used in conjunction with a 4F or 5F catheter (44%), though the difference was not statistically significant.
Although significantly fewer protection devices were used in patients in group 1 versus group 2, the number of patients with NES in the territory of the treated artery (9 in group 1 versus 7 in group 2) and the other territories (7 in group 1 versus 0 in group 2) indicates the importance of careful manipulation of the devices in the aortic arch and proximal arch vessels before protection-device placement and the CAS procedure itself. DWI after diagnostic and interventional cerebral angiography has shown that the rate of NES ranges from 15% to 23%.21–24 The high incidence of NES after diagnostic cerebral angiography by using a 4F or 5F catheter was related to the lack of systemic heparinization. Treatment with heparin or air filters resulted in an independent reduction of NES in patients undergoing diagnostic cerebral angiography from 22% to 6%.25 Thus, the catheterization technique with small and more affordably shaped catheters with adequate systemic heparinization and protection devices may reduce the incidence of thromboembolic events during CAS.
In agreement with previous findings,12 we found that increased age was the only significant risk factor for the occurrence of new DWI lesions. This may be due to a higher proportion of patients with widespread atherosclerosis.12
Most periprocedural NES on DWI do not cause obvious neurologic deficits. The clinical meaning of this silent cerebral ischemia is not fully understood. Several studies have specifically addressed the association between silent NES on DWI and neuropsychological deficits, with differing results.26–28 For example, NES after coronary angiography was associated with a decline in neuropsychological test performance.26 In contrast, this association was not observed after cardiac surgery.28 Although these differences may be due to small patient samples, the researchers suggested that NES may have a pathophysiologic role in cognitive decline in a subgroup of patients, warranting further investigation in larger populations. Although few patients who experience NES after CAS have clinically overt NES, DWI shows the overall lesion load induced by CAS. Reducing NES may, therefore, improve the outcome of patients undergoing CAS by reducing the incidence of symptomatic thromboembolic complications.
Our study has some limitations due to its retrospective design without randomization. To avoid bias, we selected consecutive patients who had undergone CAS since 2007, when the pre- and post-MR imaging protocol was applied strictly to patients planning CAS. Our institution already had a 6-year experience of CAS performed by experts in the departments of neurointervention, neurology, and neurosurgery; hence, the difference in physician experience between patients in group 1 (mainly in 2007) and in group 2 (mainly in 2008) should have had little effect on the results of this study. This retrospective study still retained a problem of the influence of diagnostic cerebral angiography performed before CAS on NES seen after CAS. However, this was the same situation in both groups, and we excluded patients with new ischemic symptoms between preprocedural DWI and the procedure and those with an exchange maneuver for carotid artery catheterization due to complicated angioarchitecture. It is difficult to expect any different significant effects on the results of NES between the 2 groups caused by diagnostic cerebral angiographies that were performed by the same neurointerventionalist in similar populations. Significant differences in the number of patients with protection devices between the 2 groups is also a main limitation of our study. Therefore, we could not interpret the results of NES in the territory of the treated artery, even though statistics showed a significance of those results. We could just mention the meaning of NES in the other territories unrelated to protection devices. Another limitation is the small number of included patients. However, despite the small sample size, the significant differences in our results are likely meaningful.
Conclusions
We found that CAS was associated with NES in the territory of the treated artery and the other territories outside this distribution. NES in the other territories arose mainly from atherosclerotic aortic arches and arch vessels proximal to the treated lesions during the manipulation of endovascular devices. We found that the carotid artery catheterization technique with a coaxial system in which a 7F or 8F catheter was used in conjunction with a 4F or 5F catheter reduced the incidence of NES in the other territories, suggesting that using a smaller more properly shaped device in the aortic arch and arch vessels is less traumatic than using a guiding catheter alone and that the former decreases the mobilization of atheromas. Routine use of the carotid artery catheterization technique using a coaxial system with a 7F or 8F catheter in conjunction with a 4F or 5F catheter in CAS may improve patient outcomes and indicates that the development of less traumatic guiding systems is necessary.
Footnotes
-
Indicates Editor’s Choices selection
-

Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 1, 2010.
- Accepted after revision March 11, 2010.
- Copyright © American Society of Neuroradiology