Abstract
BACKGROUND AND PURPOSE: Although MBs, ICH, and LI are secondary to cerebral microangiopathy, it remains unclear whether the location of subsequent ICH/LI corresponds to the previous location of MBs. We performed this study to clarify the positional relationship between recurrent ICH/LI and previously detected MBs.
MATERIALS AND METHODS: We evaluated patients with recurrent ICH/LI who had MBs, as shown on prior T2*-weighted MR imaging. We assessed retrospectively whether the location of recurrent ICH/LI corresponded to that of the prior MB. Patients with ICH were divided into the deep ICH group and the lobar ICH group, and the positional relationship between hematoma and previously detected MBs was evaluated.
RESULTS: A total of 55 patients, including 34 with recurrent ICH and 21 with recurrent LI were evaluated. Although the location of the LI corresponded to prior MBs in only 1 patient (4.8%), the location of ICH corresponded to prior locations of MBs in 21 patients (61.8%) (OR, 32.3; 95% CI, 3.86–270.3; P < .001). Among the patients with ICH, the correspondence ratio was higher in the deep ICH group (19 of 24 patients, 79.2%) than in the lobar ICH group (2 of 10 patients, 20%) (OR, 15.2; 95% CI, 2.42–95.3; P < .002).
CONCLUSIONS: The close positional association between recurrent ICH and prior MBs suggests that MBs represent hemorrhage-prone microangiopathy. In addition, different correspondence ratios between the deep ICH group and the lobar ICH group may be attributable to their different pathogenesis.
Abbreviations
- ATBI
- atherothrombotic brain infarction
- CAA
- cerebral amyloid angiopathy
- CE
- cardioembolic infarction
- CI
- confidence interval
- DWI
- diffusion-weighted imaging
- ICH
- intracerebral hemorrhage
- LI
- lacunar infarction
- MB
- microbleed
- OR
- odds ratio
MBs present as homogeneous round lesions with signal-intensity loss on gradient-echo T2*-weighted MR images. Pathologically, they represent hemosiderin deposits,1,2 associated with small-vessel disease.
Previous studies have shown that MBs are observed more frequently in patients with ICH compared with patients with ischemic stroke.3,4 Among patients with ischemic stroke, they are observed more frequently in patients with LI, which is based on small-vessel disease, compared with patients with ATBI or CE.5,6 In addition, MBs are more prevalent among patients with recurrent stroke compared with patients with their first stroke.4 Previous studies have also shown that the presence of MBs is an important risk factor for the occurrence of subsequent stroke, particularly hemorrhagic stroke.7–9
The topologic association, however, between the location of MBs and that of subsequent stroke is poorly understood. Although previous reports described the association between the hematoma and the distribution of MBs at the onset of ICH,10,11 a few case reports12,13 and several cases described in a prospective study that was performed for other purposes7,14 have reported that the subsequent ICH occurred in the same lesion in which prior MBs were detected. Moreover, to our knowledge, topologic association in patients with LI has not been reported.
This retrospective study was designed to clarify the positional association between recurrent ICH/LI and previously detected MBs in a relatively large number of patients.
Materials and Methods
Study Design and Patients
We evaluated consecutive patients with acute recurrent ICH/LI who were admitted to our hospital from June 2003 to June 2008. Among them, the patients who had asymptomatic MBs identified on 1.5T gradient-echo T2*-weighted MR imaging, which was performed at the time of the prior stroke event, were included in the study. Patients with CE, ATBI, or undetermined classification were excluded. The diagnosis of acute stroke was made on the basis of neurologic and neuroradiologic examinations. Recurrent stroke was classified into ischemic stroke and ICH, and ischemic stroke was further subclassified as ATBI, CE, and LI, according to the diagnostic criteria based on the National Institute of Neurologic Disorders and Stroke Ad Hoc Committee Classification of Cerebrovascular Disease III.15 Of the 55 patients included, 34 had recurrent ICH and 21 had recurrent LI.
The location of recurrent ICH was assessed by using CT, and the location of recurrent LI was assessed by DWI and apparent diffusion coefficient maps. We assessed, retrospectively, whether the location of recurrent ICH/LI corresponded to that of the previously detected MBs. Furthermore, patients with ICH were divided into the deep ICH group (hematoma present in the thalamus, the putamen, the pons, and the cerebellum) and the lobar ICH group (hematoma in a subcortical location), and the positional relationship between the hematoma and previously detected MBs was evaluated. There were no patients with a recurrent caudate hemorrhage in the present study. Previous antithrombotic therapy, the number of previously detected MBs, and the duration from the prior stroke to the recurrence were also evaluated in each patient. In patients with recurrent ICH, the hemorrhage volume was also evaluated. The study protocol for the chart review was approved by our institutional review board.
Vascular Risk Factors
We assessed vascular risk factors such as history of previous stroke and the presence of hypertension, diabetes mellitus, or hyperlipidemia. “Hypertension” was defined as systolic blood pressure of ≥140 mm Hg or diastolic blood pressure of ≥90 mm Hg, which were measured with an automated cuff-oscillometric device at least 2 times in the outpatient department before recurrence of stroke, or current medical treatment for hypertension. “Diabetes mellitus” was defined as a glycosylated hemoglobin Alc concentration of ≥6.5% or current use of hypoglycemic agents. “Hyperlipidemia” was defined as a low-attenuation lipoprotein cholesterol level of ≥140 mg/dL or current cholesterol-lowering therapy. We also recorded the prevalence of antithrombotic therapy before occurrence of the recurrent stroke in each patient.
Neuroradiologic Examinations
All patients were examined by using a 1.5T clinical MR imaging unit (Magnetom Symphony; Siemens, Erlangen, Germany) with a section thickness of 5 mm and a 1.5-mm gap between sections. We used axial T2*-weighted gradient-echo sequences (TR/TE, 800/26 ms; flip angle, 20°; FOV, 230 × 230; matrix, 192 × 256) to detect MBs at the onset of the prior stroke. In addition, at the onset of the recurrent stroke, we also performed axial DWI with single-shot echo-planar spin-echo sequences (TR/TE, 5300/135 ms; FOV, 196 × 261; matrix, 80 × 128; b-values, 0 and 1000/mm2) to evaluate the location of recurrent LI, and we performed axial head CT to evaluate the location and the volume of recurrent ICH. MBs were defined as homogeneous round lesions with a diameter of ≤5 mm characterized by signal-intensity loss on T2*-weighted MR images. Signal-intensity-loss lesions in the globus pallidum (which likely represented calcification) and the subarachnoid space (which likely represented adjacent pial vessels) were excluded. Intracerebral lesions were also excluded if they had a hemorrhagic component associated with tumor, arteriovenous malformation, cavernous hemangioma, or trauma.
“Corresponding” or “correspondence” was used if the location of MBs detected on prior T2*-weighted MR imaging was involved in the ICH detected on CT or the LI detected on DWI at the onset of recurrent stroke. Two of the authors (Y.S., H.N.) without detailed knowledge of the patients' clinical profiles retrospectively compared the same section of each film and determined the correspondence of MBs with subsequent stroke. In addition, we calculated the hemorrhage volume with the ABC/2 method, in which A is the greatest diameter on the largest hemorrhage section, B is the diameter perpendicular to A, and C is the approximate number of axial sections with hemorrhage multiplied by the section thickness.16
Statistical Analysis
For the cases of recurrent ICH versus LI and deep brain versus lobar ICH, the χ2 test or Fisher exact test for independence was used for comparison of sex ratio, hypertension, diabetes mellitus, hyperlipidemia, antithrombotic therapy, and correspondence between prior MBs and recurrent stroke for each group. The Student t test was used for comparison of age at the time of recurrent stroke. The Mann-Whitney U test was used for comparison of the hemorrhage volume, the number of previously detected MBs, and the time from prior stroke to the recurrence in each ICH group. P < .05 was considered significant. The Statistical Package for the Social Sciences, Version 16.0 for Windows (SPSS, Chicago, Illinois) was used for statistical analysis.
Results
Baseline Data
Of the 55 patients included in this study, 34 had recurrent ICH (25 men and 9 women) and 21 patients had recurrent LI (13 men and 8 women). The patients with ICH (median age, 69.5 years; range, 51–84 years) were younger compared with the patients with LI (median age, 72 years; range, 57–89 years; P = .020). Other demographic and clinical data are shown in Table 1.
Characteristics of patients with ICH and LI
Positional Relationship between Recurrent ICH/LI and Previously Detected MBs.
We evaluated the positional relationship between recurrent ICH/LI and previously detected MBs. In the recurrent ICH group, hematoma corresponded to the prior MBs in 21 of 34 patients (61.8%). Representative cases are shown in Fig 1. In contrast, LI corresponded to the prior MBs in only 1 of 21 patients (4.8%) in the recurrent LI group. The correspondence ratio was, therefore, higher in the recurrent ICH group than in the recurrent LI group (OR, 32.3; 95% CI, 3.86–270.3; P < .001). The number of MBs and the time from prior stroke to the recurrent stroke were equivalent between the recurrent ICH group and the recurrent LI group (Table 1).
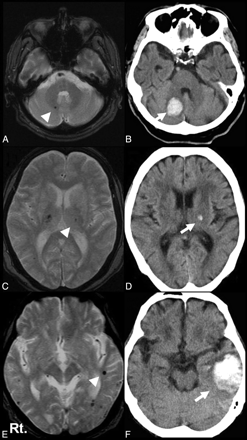
Representative cases. T2*-weighted MR image (A) and CT scan (B) in an 84-year-old patient. Recurrent right cerebellar hemorrhage (arrow) corresponds to the location of MBs detected 9 months before (arrowhead). T2*-weighted MR image (C) and CT scan (D) in an 80-year-old patient. Recurrent left thalamic hemorrhage (arrow) corresponds to the location of MBs detected 35 months before (arrowhead). T2*-weighted MR image (E) and CT scan (F) in an 85-year-old patient. Recurrent left lobar hemorrhage (arrow) corresponds to the location of MBs detected 3 months before (arrowhead).
Among the ICH group, the number of MBs was higher in the “corresponding” group (median, 16; range, 4–73) than in the “noncorresponding” group (median, 4; range, 1–49; P = .001). The hemorrhage volume and the time from prior stroke were equivalent between both groups. Vascular risk factors, antithrombotic therapy, and prior stroke subtype were also equivalent between both groups (Table 2).
Characteristics of corresponding and noncorresponding groups in patients with ICH
We also evaluated the association between the initial stroke subtype and correspondence between MB and stroke in the patients with recurrent ICH. Of the 34 patients in the recurrent ICH group, 13 patients had prior ICH and 21 had prior ischemic stroke. Among them, hematoma corresponded to the prior MBs in 8 of 13 patients with prior ICH (61.5%) and 13 of 21 patients with prior ischemic stroke (61.9%). The corresponding ratio was equivalent between the patients with prior ICH and the patients with prior ischemic stroke (P = .98).
Positional Relationship between Recurrent ICH and Previously Detected MBs in the Deep ICH Group versus the Lobar ICH Group.
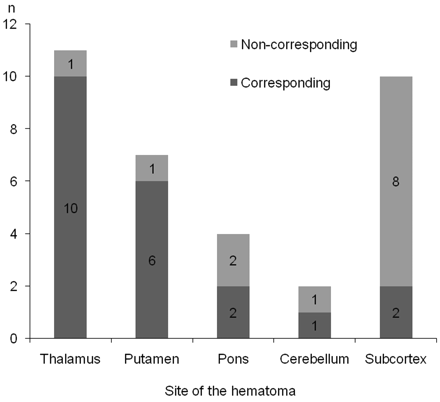
We evaluated the positional relationship between recurrent ICH and previously detected MBs for each type of hematoma (deep ICH versus lobar ICH). In the deep ICH group, hematoma corresponded to the prior MBs in 19 of 24 cases (79.2%) including 10 of 11 cases (90.0%) of thalamic hemorrhage, 6 of 7 cases (85.7%) of putaminal hemorrhage, 2 of 4 cases (50.0%) of cerebellar hemorrhage, and 1 of 2 cases (50.0%) of pontine hemorrhage (Fig 2). In contrast, in the lobar ICH group, hematoma corresponded to the prior MBs in only 2 of 10 patients (20.0%) (Fig 2). Among the patients with ICH, the correspondence ratio was higher in the deep ICH group than in the lobar ICH group (OR, 15.2; 95% CI, 2.42–95.3; P < .002). The hemorrhage volume, number of MBs, and the time from prior stroke to the recurrent ICH were equivalent between both groups (Table 3).
Correspondence of MBs in each part of the hematoma. The correspondence ratio was higher in the deep ICH group, particularly in thalamic and putaminal hemorrhage, than in the lobar ICH group.
Characteristics of deep ICH and lobar ICH groups
Among the deep ICH group, the number of MBs in the whole brain and in the gray matter (thalamus, putamen, and caudate nucleus) was higher in the corresponding group (median, 16; range, 4–56; and median, 8; range, 3–28) than in the noncorresponding group (median, 4; range, 1–11; and median, 2; range, 1–8; P = .003 and P = .015). The time from prior stroke was equivalent between both groups. Vascular risk factors and prior stroke subtype were also equivalent between both groups. The rate of antithrombotic therapy was significantly higher in the noncorresponding group than in the corresponding group (Table 4).
Characteristics of the corresponding and noncorresponding groups in the deep ICH group
Discussion
We found that the correspondence ratio was higher in patients with recurrent ICH than in patients with recurrent LI. In addition, among the patients with recurrent ICH, the correspondence ratio was higher in the deep ICH group, particularly in hemorrhage involving the putamen and thalamus, compared with the lobar ICH group.
Only a few case reports12,13 and several cases described in prospective studies performed other purposes7,14 found that the subsequent ICH occurred in the same lesion in which prior MBs were detected. The present study is the first report focusing on the positional relationship between the subsequent ICH and the prior detected MBs in a relatively large number of patients.
Pathologically, MBs represent hemosiderin deposits that result from the fragility of small vessels in conditions such as lipohyalinosis, CAA, or arteriosclerosis.1,2 The presence of MBs is closely associated with small-vessel diseases such as ICH and LI,5,6 and it has been reported to be an important risk factor for subsequent stroke, particularly hemorrhagic stroke.7–9
The difference in correspondence ratios between ICH and LI may result from the difference in topology among ICH, LI, and MBs. Previous studies showed that MBs tend to be frequently present at the site of hypertensive ICH.17,18 In contrast, MBs are seldom detected in the posterior limb of the internal capsule or the corona radiata,18 which are the frequent sites of LI. This topographic difference may explain the discrepancy of the correspondence ratios between ICH and LI. However, it remains unclear why MBs are seldom detected in the frequent sites of LI and, furthermore, why the locations of prior MBs and recurrent LI do not coincide in other brain regions, even though both MBs and LI are based on microangiopathy. The close topologic association between prior MBs and recurrent ICH but not recurrent LI indicates that MBs are a form of small-vessel disease that is bleeding-prone.
The present study also reveals that the correspondence ratio in the deep ICH group was higher than that in the lobar ICH group, though the hemorrhage volume and the number of MBs were equivalent between both groups. Our findings may support the results of the Rotterdam Scan Study that MBs in a deep or infratentorial location were associated with hypertensive or atherosclerotic microangiopathy, whereas lobar MBs were related to CAA.19 In the deep ICH group, close topologic association of prior MBs with subsequent ICH, particularly in the putamen and thalamus, suggests that subsequent hemorrhage may result from rerupture of microangiopathic vessels, such as those with lipohyalinosis in the deep brain area, which had been detected as MBs. In addition, the higher number of MBs in the deep gray matter in the corresponding group suggests that MBs in this area may be a marker of the ongoing hypertensive microangiopathy and at risk for further subsequent ICH.
In contrast, the present study reveals the lower corresponding ratio between the prior MBs and subsequent ICH in the lobar ICH group. A recent pathologic study in patients with CAA suggested that the patients with many MBs demonstrated thicker amyloid-positive vessels than those with few MBs; therefore, CAA-related hemorrhage and MBs are based on different pathologies.20 The CAA-related hemorrhage may result from rupture of amyloid-positive vessels, which are different from the vessels detected as MBs; the pathologic difference between ICH and MBs in CAA may result in the lower corresponding ratio in the lobar ICH group in the present study. However, we could not determine exactly whether the lobar hemorrhage resulted from CAA or hypertension because no patients enrolled in the present study were examined pathologically. This point is 1 of the limitations of the present study.
The other limitations should be noted. ICHs are often sizeable (particularly compared with LIs) and might, therefore, appear to coincide with a prior MBs simply because they cover a large volume of brain. It is even possible that deep ICHs, by occurring in a more confined anatomic territory than lobar ICHs, might be predisposed to coincide with prior MBs in the same territory. On the other hand, there was no difference in the hemorrhage volume between the corresponding group and the noncorresponding group overall in patients with ICH and between the deep ICH group and the lobar ICH group. Therefore, the effects of the hemorrhage volume for the corresponding ratio between the location of subsequent ICH and that of previously detected MBs may be excluded in the patients with ICH. In addition, although the corresponding ratio for patients with putamen/thalamic hemorrhages appeared to be higher than that in patients with pontine/cerebellar hemorrhages, this could be an aberration due to the small number of patients with pontine/cerebellar hemorrhage in our study. To clear up these limitations and confirm our results, we should perform prospective studies with a larger group of patients.
Conclusions
The close association between recurrent ICH and the location of previously detected MBs, especially in the putamen or thalamus, suggests that MBs represent hemorrhage-prone microangiopathy. In addition, the topologic distribution of MBs may be meaningful imaging information because the risk of subsequent ICH occurs in the same lesion in which MBs were previously detected. However, it still remains unclear whether the subsequent ICH in the location of previously detected MBs could be prevented with strict hypertension treatment or careful antithrombotic therapy, and prospective studies are needed to clarify these points.
Acknowledgments
We thank Naohisa Hosomi, MD, and Kayoko Ishihara, MD, for their advice in the drafting the manuscript and the radiologic technicians of our hospital for their acquisition of imaging data.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received October 28, 2009.
- Accepted after revision February 12, 2010.
- Copyright © American Society of Neuroradiology