Abstract
SUMMARY: Encephalopathy is an uncommon complication of childhood influenza infection, typically recognized during influenza epidemics. Imaging hallmarks include characteristic thalamic lesions, thalamic necrosis and hemispheric edema. We describe a child with acute influenza A associated necrotizing encephalopathy with MR angiographic evidence of significant cerebral vasculopathy and a hemispheric edema pattern consistent with PRES. This case reinforces that significant cerebral vasculopathy can accompany influenza infection and that influenza is a likely trigger for PRES.
Abbreviations
- ADEM
- acute disseminated encephalomyelitis
- ANE
- acute necrotizing encephalopathy
- MIP
- maximum intensity projection
- PCA
- posterior cerebral artery
- PCR
- polymerase chain reaction
- PRES
- posterior reversible encephalopathy syndrome
- RBC
- red blood cell count
- TNF
- tumor necrosis factor
- WBC
- white blood cell count
Patients with influenza infection occasionally develop neurologic deterioration, most clearly recognized during influenza epidemics. In adults, an increased incidence of stroke has been reported.1,2 In children, encephalopathy/encephalitis rarely occurs and Reye syndrome may be noted.3–6 In most reported encephalopathy/encephalitis cases, CT or MR imaging demonstrate areas of abnormality in the thalami and occasionally the brain stem, with diffuse or focal areas of abnormality or edema in the cerebral hemispheres and cerebellum.5–7 Transient/reversible lesions are reported in the thalami, centrum semiovale, and splenium of the corpus callosum.8 Postmortem reports have typically described areas of brain edema or congestion. In approximately 20% of affected children, a severe form occurs with characteristic bilateral thalamic destructive lesions, often with accompanying multifocal lesions in the brain stem, cerebellum, and hemispheric white matter, labeled ANE.3,4
PRES is seen in association with infection,9 and recently, PRES has been reported accompanying influenza A infection in an adult.10 Here, we report a case of a child with acute influenza-associated encephalopathy, cerebral vasculopathy, and cerebral hemispheric abnormality with a pattern consistent with PRES.
Case Report
A previously healthy 3-year-old girl was admitted to an outside institution after 1 day of fever, cough, rhinorrhea, fatigue, and lethargy. The patient soon became unresponsive and began posturing. On admission, CSF analysis showed the following values: 1 RBC, 2 WBC with 100% polymorphonuclear leucocytosis; protein, 33 mg/dL; glucose, 83 mg/dL. Blood pressure was normal. CT showed bilateral thalamic lesions with possible petechial hemorrhages. Antiepileptic medications were administered on seizure-suspicious activity. The patient was transferred to our institution intubated.
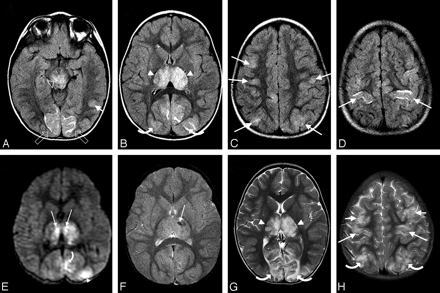
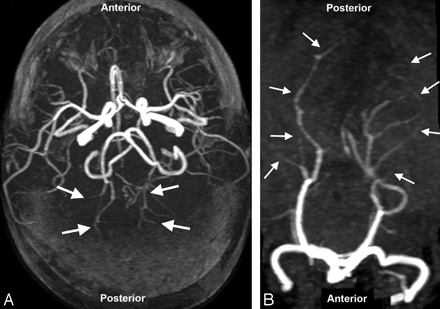
On transfer, the child remained neurologically unchanged with blood pressure 113/83 mm Hg and fever of 39.4°C (102.9°F). MR imaging on admission redemonstrated bilateral thalamic lesions with slight restricted diffusion, evidence of adjacent petechial thalamic hemorrhage as well as cortical and subcortical white matter abnormalities in the occipital region, occipital-parietal junction, and frontal lobes bilaterally (Fig 1 A–F). MR angiography demonstrated vasculopathy with foreshortening, irregularity, and pruning of the posterior cerebral arteries bilaterally (Fig 2). MR venography findings were normal.
MR images obtained immediately on transfer (A–F) and on follow-up at 5 days (G–H). A–D, Axial FLAIR MR images obtained immediately on transfer demonstrate signal-intensity abnormality in the thalami bilaterally (arrowheads), characteristic of influenza-associated encephalopathy. Also present is abnormal signal intensity in the cortex and subcortical white matter of the occipital poles (open arrows), occipital-parietal junction (curved arrows), and posterior frontal lobes (arrows) bilaterally, left inferior temporal-occipital junction (short arrow), and midposterior cerebellar hemispheres (not shown), consistent with PRES vasogenic edema. The edema is greatest in the occipital-parietal region (curved arrows). Additional focal lesions are present in the midbrain (open arrowhead), pons, and middle cerebellar peduncle. E, Axial diffusion-weighted MR image demonstrates small foci of restricted diffusion in the thalami bilaterally (arrows) consistent with early necrosis of influenza-associated ANE. A small focus of restricted diffusion is present in the medial left occipital-parietal junction (small curved arrow) with a second likely present more peripherally in areas of PRES vasogenic edema (arrowhead). These regions demonstrate true restricted diffusion on the accompanying apparent diffusion coefficient map. F, Axial gradient-echo T2* MR image demonstrates the small focus of petechial hemorrhage in the left thalamus (arrow) just lateral to the left thalamic restricted diffusion. G–H, Follow-up T2-weighted MR images obtained on day 5 demonstrate the more typical features of PRES vasogenic edema in the cortex and white matter of the occipital-parietal and frontal-parietal junctions (curved arrows) and frontal lobes (arrows), along with continued demonstration of the bilateral thalamic lesions (arrowheads).
3D time-of-flight MR angiogram sequence also obtained immediately on transfer. A, Collapsed MIP view demonstrates marked vessel foreshortening and irregularity of the PCAs bilaterally (arrows), likely related to reduced flow from peripheral primary or secondary vasculopathy. Anterior and middle cerebral arteries appear more normal with questionable distal branch foreshortening and irregularity. B, Focused rotated MIP view of the posterior circulation better demonstrates marked PCA distal branch irregularity with areas of vessel narrowing and dilation (arrows).
Nasal swab/washing obtained on admission was positive for influenza A (H1N1 seasonal) by PCR assay but negative for influenza B and respiratory syncytial virus. Repeat CSF analysis remained unremarkable with the following values: RBC, 2; WBC, 1 (lymphocytes, 33%; polymorphonuclear leukocytes, 7%); protein, 77 mg/dL; glucose, 70 mg/dL, including negative evaluation for enterovirus, Herpes Simplex Virus, and Human Herpes Virus-6 by PCR assay. Results of admission blood and urine cultures remained normal, including the PCR assay for Epstein-Barr virus. A moderate decline in platelet count (to 194 × 103 mL−1; baseline, 376 × 103 mL−1) and left lower lobe patchy lung attenuation suggesting atelectasis were present on day 2, but total bilirubin and serum creatinine concentrations remained normal, with true multiorgan dysfunction syndrome not technically present.
In light of the above clinical results with documentation of influenza A infection, the bithalamic lesions were interpreted as specific for influenza A encephalopathy with the occipital, occipital-parietal junction, and frontal vasogenic edema considered consistent with PRES. A secondary diagnosis of ADEM was entertained, but the time of onset was technically too acute and the cortex imaging features were considered uncharacteristic.
On further neurologic decline, repeat MR imaging demonstrated progression of the PRES vasogenic edema (Fig 1G–H) with focal areas of restricted diffusion in the cortex and extensive stippled cortical enhancement. Restricted diffusion was more apparent in the bithalamic lesions and was also present in the centrum semiovale.
The child ultimately stabilized neurologically, however with marked neurologic impairment, and was transferred to a rehabilitation center.
Discussion
To our knowledge, this is the first report of childhood acute influenza-associated encephalopathy with MR angiographic evidence of vasculopathy and an accompanying hemispheric edema pattern consistent with PRES. While the cause of PRES and influenza-associated encephalopathy/encephalitis are uncertain, coincident identification in a child reinforces the observation that PRES can be triggered by influenza and suggests a common underlying mechanism for the observed brain changes.10
Acute influenza-associated encephalopathy/encephalitis usually affects children younger than 5 years of age, with neurologic deterioration occurring 1–3 days after onset of influenza symptoms (typically H3N2 or H1N1).5 Moderate/severe brain edema is commonly reported at postmortem,3,4,10,11 and CT/MR imaging findings are abnormal in up to 70% of cases.5,6 Imaging studies have demonstrated bilateral thalamic lesions, variable diffuse/focal hemispheric edema, and reversible lesions in the thalami, splenium, and centrum semiovale.6,8,12,13 Older case reports have demonstrated evidence of cerebral vasculopathy or vasculitis.4,14,15 In approximately 20% of children, the more severe form is encountered, with bilateral thalamic necrosis and restricted diffusion recently termed “ANE.”3,4 Delayed postinfectious presentations also occur, similar to those in ADEM.16
A plasma/CSF inflammatory cytokine response is consistently noted (TNF-α, interleukin-6 and TNF-receptor 1) in influenza-associated encephalopathy/encephalitis,4 and evidence of multiorgan dysfunction is observed.5 Spinal fluid may demonstrate elevated protein in encephalopathy, with encephalitis diagnosed when a pleocytosis (>8 WBC dL−1) is present.4,5 Histology has demonstrated lymphocyte trafficking in the perivascular spaces and meninges without distinct parenchymal accumulation.17,18 Endothelial infection by influenza has been demonstrated extensively in vitro,19–21 and while viral genetic material has been identified in brain parenchyma,11,15,20,22 infectious virus has rarely been recovered.11,20
PRES has been reported in association with severe infection, sepsis, or shock.9,23 A characteristic watershed-like pattern of reversible vasogenic edema is most commonly identified in the cerebral hemispheres and cerebellum, with focal edema also seen in the basal ganglia, thalami, pons, and medulla.23 Restricted diffusion, hemorrhage, and stippled cortical enhancement can occur in areas of PRES vasogenic edema.23 Cerebral vasculopathy with reversible vasoconstriction and vessel irregularity have been documented at catheter and MR angiography.23
The mechanism of PRES has not been determined. Endothelial activation, T-cell activation, and a pronounced inflammatory cytokine response are central to most PRES-related conditions.24 Hypoperfusion has been demonstrated as well (MR/CT perfusion, single-photon emission tomography).23,24 At histology, brain vasogenic edema and perivascular lymphocytic trafficking have been documented.23 Recent identification of endothelial activation, T-cell trafficking, and vascular endothelial growth factor up-regulation in transplant-associated reversible encephalopathy suggests hypoxemia-induced vasogenic edema.25
Our case demonstrates clinical and imaging features most consistent with the acute necrotizing form of influenza encephalopathy, including typical age and rapidity of symptom onset, disease course, absent CSF pleocytosis, presence of thalamic lesions with restricted diffusion and hemorrhage, and areas of hemispheric edema. Delayed restricted diffusion in the centrum semiovale was also present in our case, similar to that in a prior case report.8 The hemispheric cortical/subcortical white matter edema pattern in our patient is characteristic of PRES, which, in this case, occurred coincident with the acute influenza A infection. The initial MR imaging appearance was slightly unusual for PRES (predominant cortical appearance), likely due to very early evaluation during the acute process. Follow-up imaging at 5 days demonstrated a more typical PRES appearance (more extensive vasogenic edema in the occipital-parietal cortex and adjacent white matter, foci of cortical restricted diffusion, presence of stippled cortical enhancement). Vasculopathy as has been reported in PRES23 and the foreshortened and irregular posterior cerebral arteries present at MR angiography in our patient are consistent with reduced cerebral blood flow due to primary or secondary vasculopathy.
Repeat demonstration of influenza-associated vasculopathy as seen in our patient suggests the observation might be common, possibly reflecting direct endothelial viral involvement or a response to the viral-triggered immune-related systemic process. In addition, coincident demonstration of PRES and influenza encephalopathy, particularly in the setting of normotensive blood pressure, reinforces a likely influenza-associated mechanism for PRES. In children, the observed vasculopathy may be responsible for both the destructive and reversible lesions seen in influenza-associated encephalopathy/encephalitis. In adults, influenza-associated vasculopathy could contribute to the systemic complications of influenza infection, including the increased incidence of stroke and myocardial infarction.
References
- Received June 24, 2009.
- Accepted after revision August 14, 2009.
- Copyright © American Society of Neuroradiology