Abstract
SUMMARY: Rituximab is a monoclonal antibody that was first approved by the FDA as an antineoplastic agent designed to treat B-cell malignancies. This article will review the mechanism of action and clinical role of this anti-B-cell agent.
Abbreviations
- FDA
- US Food and Drug Administration
- FSE
- fast spin-echo
Rituximab is a monoclonal antibody that targets CD20, a specific B-cell surface antigen. Rituximab (Rituxan; Biogen-IDEC/Genentech, South San Francisco, California) was the first monoclonal antibody approved for the treatment of non-Hodgkin lymphoma.1 In 1997, the FDA approved rituximab for the treatment of refractory low-grade lymphoma.2 This medication has since been used for the treatment of a number of CD20-positive B-cell malignancies.3,4 The selectivity of the drug for B-cells led to further investigations involving autoimmune B-cell−driven diseases, including rheumatoid arthritis.5 Rituximab has since been approved for the treatment of rheumatoid arthritis by the FDA.
Mechanism of Action
Rituximab is a chimeric murine/human monoclonal immunoglobulin G1 antibody that targets CD20, which is a B-cell differentiation marker.6 CD20 is a cell-surface marker specifically found on pre-B and mature B lymphocytes and is not found on other cell types or free in circulation.7 The only binding site for rituximab is CD20 on B-cells. The binding of rituximab to cell surface CD20 located on the B lymphocytes results in destruction of the lymphocyte by 3 potential mechanisms, including complement-dependent cytotoxicity, stimulation of apoptosis, or antibody-dependent cytotoxicity (Fig 1.) Complement-mediated cytotoxicity most likely is the dominant mechanism in vivo.8
Schematic illustration of the mechanism of action of rituximab. The antibody labels B lymphocytes, which have the CD20 cell marker. These cells are then killed by 1 of 3 mechanisms: antibody-dependent cytotoxicity, complement-dependent cytotoxicity, or stimulation of apoptosis. Illustration by Priya A. Rajdev.
Clinical Indications
Rituximab has been approved by the FDA for various B-cell non-Hodgkin lymphomas (clinical case: Figs 2 and 3) and rheumatoid arthritis. There are multiple off-label uses, including chronic lymphocytic leukemia, systemic lupus erythematosus, multiple sclerosis, autoimmune hemolytic anemia, posttransplant lymphoproliferative disorder, graft-versus-host disease, pemphigus vulgaris, chronic immune-mediated thrombocytopenia, and Evans Syndrome.
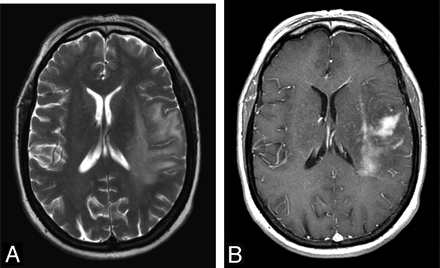
A 68-year-old woman presented with clumsiness, dropping things from her right hand, difficulty speaking, and right facial droop. A, T2-weighted axial FSE image of the brain shows patchy areas of increased T2-signal-intensity edema surrounding a low T2-signal-intensity lesion at the left temporal and frontal parietal lobes. B, T1-weighted postcontrast axial spin-echo image of the brain shows irregular and patchy enhancement of the lesion. Surrounding edema is seen as a low T1 signal intensity. A biopsy of the mass was performed, and CD20-positive B-cell lymphoma was diagnosed.
The patient was admitted and received chemotherapy with methotrexate, vincristine, and rituximab. Follow-up MR imaging was performed 2 cycles into her chemotherapy. A, T2-weighted axial FSE image shows only residual edema as high T2 signal intensity in the deep white matter of the left frontal parietal lobes. B, Enhanced T1-weighted axial spin-echo image shows resolution of the enhancing mass.
Administration
Rituximab is a prescription drug administered intravenously. The half-life is relative to the dose and number of doses administered but ranges between 1.6 and 20 days.9 The medication remains detectable in the serum for ≤6 months after a single infusion. B lymphocyte depletion typically occurs by 2 weeks and recovery begins at 6 months and continues until 12 months.10,11 Patients may continue to have subtle abnormalities in B lymphocyte populations for several years following treatment.
Side Effects
There are several specific US boxed warnings on the package insert12:
Transfusion Reaction.
Severe transfusion reactions resulting in anaphylaxis characterized by fever, hypotension, bronchospasm, urticaria, and angiodema. Eighty percent of cases seen with the first infusion.
Progressive Multifocal Leukoencephalopathy.
Progressive multifocal leukoencephalopathy is a serious central nervous system infection due to the JC virus, which typically occurs within 12 months of the first infusion. Diagnosis requires MR imaging and spinal tap.13
Skin.
Severe mucocutaneous reactions resembling Stevens-Johnson reactions or toxic epidermal necrolysis.
Tumor Lysis Syndrome.
Tumor lysis syndrome is caused by tumor cell death typified by hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. It may be complicated by acute renal failure.
Infections.
Patients are susceptible to opportunistic infections, including pneumocystis pneumonia.14 Patients are also prone to severe viral infections with parvovirus B19, varicella, cytomegalovirus, or herpes simplex virus.15
Hepatitis.
Reactivation of hepatitis B has been reported, resulting in fulminant hepatitis and hepatic failure. Screen high-risk patients before the initiation of therapy.
Economic Issues
Rituximab costs approximately $630 for a 10-mg vial. Typical dosing regimens range from 375 to 500 mg/m2 for 1–4 doses, depending on the indication but can be as high as 1000 mg for rheumatoid arthritis.
Clinical Issues
Patients require periodic laboratory follow-up with complete blood count, comprehensive metabolic panel, immunoglobulin levels, and lymphocyte counts/subpopulations. This is dependent on dose, indication, and duration of therapy. Any new neurologic or skin changes need to be evaluated by a physician.
References
- Received March 10, 2010.
- Accepted after revision March 12, 2010.
- Copyright © American Society of Neuroradiology