Abstract
SUMMARY: Clopidogrel is an inhibitor of platelet aggregation, indicated for the prevention of ischemic stroke and in-stent thrombosis. However, it has a number of drawbacks, including an increased risk of hemorrhage; a clinical effect that is slow in onset and irreversible; a genetically determined variability in its clinical potency; and interactions with other commonly administered drugs.
ABBREVIATIONS
- Act-Met
- active metabolite of clopidogrel
- ADP
- adenosine diphosphate
- AHA
- American Hospital Association
- GI
- gastrointestinal
- GPIIb/IIIa
- glycoprotein IIb/IIIa
- PPI
- proton pump inhibitor
- VWF
- von Willebrand factor
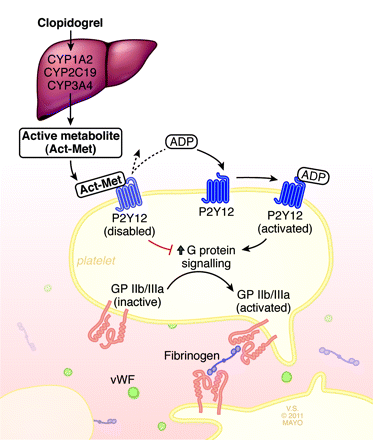
Clopidogrel (Plavix, Bristol-Myers Squibb, Washington, DC; Sanofi Aventis, Bridgewater, New Jersey) is a prodrug of the thienopyridine family, which has little, if any, platelet inhibitory effect in its original state. Its active form (Act-Met) is produced by metabolism of the prodrug through the cytochrome P450 system of the liver, most particularly by the enzyme CYP2C19.
Act-Met permanently and irreversibly disables the G protein−coupled platelet receptor known as P2Y12. P2Y12, normally activated by ADP, brings about conformational change of the surface molecule GPIIb/IIIa. This conformational change increases the affinity of GPIIb/IIIa for the divalent bridging molecules fibrinogen and VWF, thereby allowing platelet aggregation (Fig 1). As such, activated clopidogrel-mediated disabling of this G protein−coupled receptor leads to diminished aggregation of platelets.
Production of clopidogrel active metabolite and its effect on platelet aggregation. Clopidogrel is metabolized in the liver by cytochrome P450 enzymes, producing Act-Met. Act-Met irreversibly binds and disables the platelet receptor P2Y12. ADP-induced P2Y12 activation causes downstream G-protein signaling, which in turn results in conformational change and activation of the platelet receptor GPIIb/IIIa. This activated GPIIb/IIIa receptor can bind to similarly activated receptors on other platelets via divalent bridging molecules such as VWF and fibrinogen, resulting in platelet aggregation.
Clinical Indication
According to the most recent AHA/American Stroke Association Council “Guidelines for the Prevention of Stroke,”1 clopidogrel is indicated to prevent noncardioembolic stroke in patients with known atherosclerotic disease, though it is not definitely superior to aspirin or aspirin/dipyridamole for this purpose. The same guidelines asserted that, currently, insufficient evidence exists to recommend its administration in the acute treatment of ischemic stroke.
Clopidogrel is familiar to the neurointerventionalist in the setting of intracranial or extracranial carotid/vertebral stent placement. The evidence to support its use in this circumstance is primarily extrapolation from trials of coronary arterial stent placement.
Administration
Plavix is commercially available only in an oral form. Trials validating its effectiveness in the setting of secondary prevention of ischemic stroke have used once-daily doses of 75 mg. This dose is expected to result in a steady-state effect on platelet aggregation after approximately 5–6 days.2 This dose is, however, arbitrary, and may not be sufficient to produce optimal platelet inhibition in all patients.
In the acute setting of emergent arterial stent placement, the optimal loading dose has not been clearly established in the cardiology literature; there is some evidence to suggest that 600- or 900-mg loading doses are more efficacious than the traditionally used 300-mg loading dose.3 A loading dose of 900 mg may result in the same level of platelet inhibition after just 2 hours as a loading dose of 300 mg achieves after 6 hours.4
Side Effects
The most clinically significant side effect of clopidogrel is hemorrhage. The observed risk with clopidogrel is significantly lower than that observed with aspirin for GI bleeding (2% versus 2.66% for clopidogrel and aspirin, respectively) and is similar for intracranial hemorrhage (0.31% versus 0.42% for clopidogrel and aspirin, respectively).5 Other side effects include pruritus and rash6 and, rarely, thrombotic thrombocytopenic purpura.7
Poor Responders
It is believed that a subset of approximately one-third of patients are “poor responders” to clopidogrel,8 with diminished in vitro reduction of ADP-induced platelet aggregation and proved increased risk of in-stent and native arterial thrombosis.9
The most important intrinsic determinant of this variable response is likely to be related to polymorphisms within the genes that regulate CYP2C19 activity,10 and these are particularly common in Asian populations.11
A further likely intrinsic determinant is variable intestinal absorption, thought to be related to polymorphisms of the ABCB1 gene.10
There are various commercially available tests of in vitro platelet function, including ADP-stimulated platelet aggregation and vasodilator-stimulated phosphoprotein phosphorylation. Their clinical utility in determining clopidogrel clinical response and dosing is controversial.
Interactions
With regard to pharmacokinetic interactions, the most important are with drugs that inhibit CYP2C19 activity and thereby reduce conversion of the prodrug to its active form. The most important of these is the PPI group; these medications are both ubiquitous (available over-the-counter in many countries) and are often deliberately coadministered to patients receiving clopidogrel to reduce the risk of GI hemorrhage.
There is definite reduced ex vivo inhibition of platelet aggregation by clopidogrel when PPIs are coadministered.12 However, the current position of the AHA is that there is no conclusive evidence that this translates to an increased risk of clinically significant thrombosis.13
Strategies suggested to minimize PPI-induced inhibition include substituting the less inhibitory pantoprazole for omeprazole,14 and spacing clopidogrel and PPI doses 12 hours apart from each other.15 The entire subject remains controversial.
Economic Issues
The cost of Plavix was estimated at US $103 per month in 2007 inflation-adjusted terms, compared with US $6 per month for aspirin, in a study comparing the cost-effectiveness of the 2 agents.16 This does, however, compare favorably with the newer thienopyridines, and with the direct GPIIb/IIIa antagonists. Clopidogrel is protected by US patent until May 201217; generic versions may further reduce the cost of treatment.
Summary
Clopidogrel remains a front-line drug in the prevention of arterial thrombosis in high-risk patients, such as those with arterial stents and those with atherosclerotic disease. However, its problematic pharmacokinetics mean that it may be supplanted by the newer P2Y12 antagonists, which have fewer clinically significant drug interactions, a faster onset, reversibility, and more predictable and greater clinical potency.
Clopidogrel summary
Footnotes
-
Disclosures: David Kallmes—UNRELATED: Grants/Grants Pending: ev3, Penumbra, MicroVention, Sequent, NFocus, Payment for Development of Educational Presentations: ev3, CareFusion, Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: MicroVention.
References
- © 2011 by American Journal of Neuroradiology