Abstract
BACKGROUND AND PURPOSE: Endovascularly coiled intracranial aneurysms are increasingly being followed up with noninvasive MRA imaging to evaluate for aneurysm recurrences. It has not been well-established which MRA techniques are best for this application, however. Our aim was to prospectively compare 4 MRA techniques, TOF and CE-MRA at 1.5T and 3T, to a reference standard of DSA in the evaluation of previously endovascularly coiled intracranial aneurysms.
MATERIALS AND METHODS: Fifty-eight subjects with 63 previously coiled intracranial aneurysms underwent all 4 MRA techniques within 8 days of DSA. There were 2 outcome variables: coil occlusion class (class 1, complete; class 2, dog ear; class 3, residual neck; class 4, aneurysm filling) and change in degree of occlusion since the previous comparison. Sensitivity and specificity were computed for each MRA technique relative to the reference standard of DSA. Differences among the MRA techniques were evaluated in pair-wise fashion by using the McNemar test.
RESULTS: For the detection of any aneurysm remnant, the sensitivity was 85%–90% for all MRA techniques. Sensitivity dropped to 50%–67% when calculated for the detection of only the class 3 and 4 aneurysm remnants, because several class 3 and 4 remnants were misclassified as class 2 by MRA. CE-MRA at 1.5T and 3T misclassified fewer of the class 3 and 4 remnants than did TOF-MRA at 1.5T, as reflected by the significantly greater sensitivity for larger aneurysm remnants with CE-MRA relative to TOF-MRA at 1.5T (P = .0455 for both comparisons).
CONCLUSIONS: CE-MRA is more likely than TOF-MRA to classify larger aneurysm remnants appropriately. We recommend performing both CE-MRA and TOF-MRA in the follow-up of coiled intracranial aneurysms and at 3T if available.
Abbreviations
- ASNR
- American Society of Neuroradiology
- CAQ
- Certificate of Added Qualification
- CE
- contrast-enhanced
- DSA
- digital subtraction angiography
- LOC
- locations
- MIP
- maximum intensity projection
- MRA
- MR angiography
- SNR
- signal intensity–to noise ratio
- SPGR
- spoiled gradient-recalled echo
- TOF
- time-of-flight
Intracranial aneurysms remain a significant source of neurologic morbidity and mortality. Their management options continue to be open surgery, endovascular platinum microcoil embolization, or observation, depending on several factors. Endovascular coiling has become a mainstay, if not first-line therapy, for many aneurysms, particularly following the results of the International Subarachnoid Aneurysm Trial.1 However, imaging follow-up is necessary after endovascular coiling because of frequent incomplete occlusion, a 10%–40% rate of coil compaction or aneurysm regrowth,2,3 and the risk of aneurysm rupture following incomplete coiling or aneurysm recurrence.4 Catheter cerebral angiography (DSA) has been the reference standard for coiled aneurysm follow-up, but its invasiveness, risk of complications, required time, and expense are drawbacks.5
MRA has shown promise in detecting aneurysm remnants or recurrences after endovascular coiling. However, MRA techniques vary in quality with imaging equipment and imaging site, they change or develop with time, and their accuracy relative to conventional angiography and to each other has not been strongly established. There remains ambiguity over the overall performance of MRA relative to DSA in the depiction of coiled aneurysm remnants and in the relative performance of TOF-MRA, bolus gadolinium CE-MRA, and MRA performed at the field strengths of 1.5 T versus at 3T, with different groups reporting conflicting results.6–19
We wished to prospectively study the performance of MRA relative to DSA for the clinical indication of the follow-up of coiled intracranial aneurysms, and more specifically, we wished to study the relative performance of the 2 major types of MRA at 2 popular main magnetic field strengths, 1.5 and 3T. We present the results of a prospective clinical trial comparing the accuracies of 4 different MRA techniques, TOF and CE-MRA both at 1.5T and at 3T, by using DSA as the standard of reference in the follow-up of coiled intracranial aneurysms.
Materials and Methods
Patient Selection
This study was approved by our institutional review board for human studies and is Health Insurance Portability and Accountability Act−compliant. Informed consent was obtained for all subjects. During a 3-year period, 58 subjects with a total of 63 previously endovascularly coiled intracranial aneurysms were prospectively enrolled when they returned for follow-up conventional angiography. Potential subjects with contraindications to MR imaging or intravenous gadolinium were excluded. Those with previous intracranial aneurysm clipping or other contraindications to 3T MR imaging were excluded. Those who could not undergo both follow-up conventional angiography and MRA within 10 days were also excluded.
For this follow-up imaging, some patients underwent MRA before conventional angiography and some received conventional angiography before MRA. However, patients were also excluded if they had undergone recoiling at follow-up angiography before undergoing follow-up MRA.
MR Imaging
Subjects underwent 4 separate MRA examinations in the same sitting. TOF-MRA and gadolinium bolus CE-MRA at both 1.5T and 3T field strengths have been in standard clinical use at our institution. The first one-third of patients randomly underwent both 1.5T examinations followed by both 3T examinations or vice versa. Because of the potential for venous contamination of TOF examinations from previous gadolinium injections, the last two-thirds of patients underwent the TOF-MRA examinations at both field strengths (1.5T and 3T), followed by the CE-MRA examinations at both field strengths, with the order of field strength varied randomly. MRA examination parameters are given in Table 1. Zero-fill interpolation reconstruction was used for all examinations. The TOF examinations used multiple overlapping thin-slab acquisitions. Following timing series test boluses with 2 mL of gadodiamide (Omniscan; GE Healthcare, Chalfont St. Giles, United Kingdom), the gadolinium bolus examinations were each performed with injections of 25 mL of gadodiamide at 3 mL/s; each then was followed by a saline flush. The CE-MRA examinations used elliptic centric view-order sampling of k-space.
MRA scanning parameters
MR imaging systems used were 1.5T and 3T Signa scanners (GE Healthcare, Milwaukee, Wisconsin). An 8-channel phased-array head coil or bird cage coil was used at 1.5T, and an 8-channel phased-array head coil was used at 3T.
Catheter Angiography
Standard clinical catheter DSA was performed by 1 of 2 neuroradiology CAQ-certified interventional neuroradiologists (H.J.C., D.F.K., both with 15 years of experience). In general, 5F diagnostic angiography catheters were placed in the internal carotid artery or the vertebral artery, and multiplanar DSA was performed. In most cases, 3D rotational angiography was performed as well.
Image Analysis
All MRA scans that were obtained were evaluated—that is, no patient's scan was excluded from the analysis. Coiled aneurysms were independently evaluated from MRA examinations at an Advantage Workstation 4.3 (GE Healthcare) by 2 CAQ-certified diagnostic neuroradiologists (J.H., T.J.K., with 15 and 5 years of experience, respectively), who had knowledge from prior DSA reports of where each coiled aneurysm was to be expected. Source images and multiplanar reformatted images (in 3 orthogonal planes) were reviewed for each case. FOV, section thickness, and windowing were adjustable and chosen interactively at the discretion of the radiologist. Patient examinations were divided into 4 chronologic groups during the course of the study. For reviewing purposes, within each of these 4 groups, patient order of review and particular MRA technique were randomized. This randomization was performed to minimize readers’ recall of details of particular aneurysms from 1 MRA technique to the next, yet reviewing cases in 4 chronologic groups allowed ongoing data analysis throughout the trial. Readers were blinded to concurrent conventional angiography results. Following independent review, these readers adjudicated those data points for which there was any disagreement.
Similarly, coiled aneurysms were independently evaluated from digitally displayed conventional angiography examinations by 2 CAQ-certified interventional neuroradiologists (H.J.C., D.F.K.). These readers were blinded to the results of concurrent MRA examinations. Following independent review, these readers adjudicated those data points for which there was any disagreement.
Readers for both the MRA examinations and the conventional angiography examinations recorded 2 ordinal characteristics for each coiled aneurysm: coil occlusion class (after the scheme of Roy et al20: 1 = complete occlusion, 2 = dog ear, 3 = residual neck, 4 = aneurysmal filling) and change since the most recent comparison catheter angiogram (better, same, or worse in terms of degree of coil occlusion). These outcome variables are necessarily subjective to some degree, but application of these scales was resolved in adjudication sessions on a case-by-case basis. Previous comparison angiograms were reviewed digitally or on film, depending on availability.
Statistical Analysis
Descriptive statistics were reported for patient age and sex and imaging examination details. Using the adjudicated catheter angiogram results as the reference standard and for both outcome variables of coil occlusion class and change since comparison DSA, we calculated the sensitivity and specificity of each of the 4 MRA techniques. This was done by combining the ordinal outcome variables into binary outcome variables so that these measures of diagnostic test performance could be computed. Specifically, test performance computations were performed first by grouping any aneurysm-remnant designation from the MRA readings together (ie, grouping together aneurysm-remnant classes 2–4) and then by grouping the larger class 3 and 4 aneurysm remnants together. Thus, the “positive” test result was evaluated in 2 ways for coil occlusion class. For the outcome variable of change since comparison DSA, “same” and “better” were grouped together as the “negative” test result, and “worse” was considered to be the “positive” test result. Sensitivities and specificities were estimated along with 95% exact binomial confidence intervals to assess the performance of tests. Comparisons of test performance (ie, sensitivities and specificities) among the 4 MRA techniques were performed in a pair-wise manner by using the McNemar test.
Weighted κ values were also calculated for the assessment of interobserver agreement; between the 2 MRA readers and between the 2 catheter angiogram readers. For weighted κ values, the measures of interobserver agreement, <0.40 is usually considered poor agreement, 0.4–0.6, fair; 0.6–0.75, good; and >.75, excellent. All of the analyses were performed by using SAS Version 9 software (SAS Institute, Cary, North Carolina). All of the tests were 2-sided, and P values < .05 were considered statistically significant.
Results
Fifty-eight subjects with 63 total previously coiled intracranial aneurysms were enrolled. The subjects’ mean age was 59.3 years (range, 38–77 years). Forty-five were women and 13 were men. Their aneurysms were evaluated with conventional angiography at a median of 369 days after endovascular coiling (interquartile range, 210–463 days; range, 155-2529 days). There was a median 1-day interval between this follow-up conventional angiography and follow-up MRA (interquartile range, 1–2 days; range, 0–8 days).
Aneurysm locations were as follows: 29 (46%) of the internal carotid arteries, 18 (29%) of the posterior circulation, 11 (17%) of the anterior cerebral arteries, and 5 (8%) of the middle cerebral arteries. By our reference standard of adjudicated DSA results, 23 (37%) aneurysms were Roy et al20 class 1, 16 (25%) were class 2, 16 (25%) were class 3, and 8 (13%) were class 4. Six (10%) were improved from comparison DSA, 38 (60%) were unchanged, and 19 (30%) were worse in appearance.
Sensitivity and specificity for both outcome variables for all 4 MRA techniques are presented in Figs 1 and 2. For the detection of any aneurysm remnant (classes 2, 3, or 4), sensitivities were 90%, 85%, 88%, and 90% for 1.5T TOF, 1.5T CE, 3T TOF, and 3T CE-MRA, respectively. These sensitivities dropped to 50%, 67%, 50%, and 67%, respectively, when calculated for the detection of only the grouped class 3 and 4 aneurysm remnants. These drops in sensitivity can be explained by the fact that there were 11 class 3 remnants and 2 class 4 remnants by DSA, which were not generally missed as remnants but were underclassified by MRA (eg, class 3 remnants misclassified as class 2).
MRA technique sensitivity for any aneurysm remnant, larger (class 3 or 4) remnants, and the detection of growth since previous DSA; 95% confidence intervals are shown.
MRA technique specificity for any aneurysm remnant, larger (class 3 or 4) remnants, and the detection of growth since previous DSA; 95% confidence intervals are shown.
Specificities of these 4 MRA techniques for any aneurysm remnant were 52%, 65%, 52%, and 64%, respectively. These specificities improved to 85%, 84%, 85%, 87%, respectively, for the detection of only the grouped class 3 and 4 aneurysm remnants. Regarding the detection of any aneurysm growth since previous comparison angiograms, sensitivities for these MRA techniques were 28%, 28%, 33%, and 39%, respectively, and specificities were 93%, 95%, 98%, and 95%, respectively.
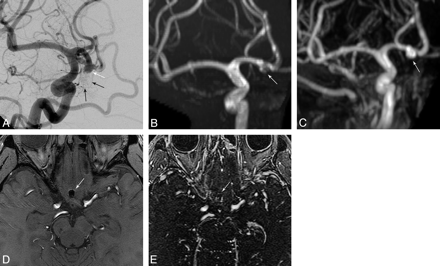
There was no statistically significant difference in sensitivity among MRA techniques for the outcome of detection of any aneurysm remnant class (Table 2). However, there was statistically significant improved sensitivity for detection of the larger class 3 or 4 aneurysm remnants with CE-MRA at both 1.5T and 3T compared with TOF-MRA at 1.5T (P = .0455 for both, Table 2), explained by several more underclassifications of aneurysm remnants by TOF-MRA at 1.5T. An example of this underclassification with TOF-MRA is given in Fig 3. There was a nonsignificant trend toward improved sensitivity for class 3 and 4 aneurysm remnants with CE-MRA at 1.5T compared with TOF-MRA at 3T (P = .1025). There was no statistically significant difference in specificity among the MRA techniques for the outcome of aneurysm remnant class or for change since previous angiography. There was no statistically significant difference in sensitivity among MRA techniques for the outcome of change since the previous angiography (Table 2). With our standard clinical scanning protocols, the location of the coil masses was generally much better identified with TOF-MRA (Fig 3).
Pair-wise comparisons of sensitivity and specificity of MRA techniques
DSA (A), 3T TOF-MRA MIP (B), 3T CE-MRA MIP (C), 3T TOF-MRA source image (D), and 3T CE-MRA source image (E). A class 4 coiled anterior communicating artery remnant/recurrence (white arrows in A, B, and C) is better depicted as a large remnant with CE-MRA (C) than with TOF-MRA (B). Digitally subtracted coils are seen in A (black arrows). However, by using our clinical scanning protocols, localization of the coil mass is improved with TOF-MRA (D, white arrow) relative to CE-MRA (E). This may be related to better visualization of the surrounding brain tissue with TOF than with CE-MRA because of a shorter TR and higher flip angle of the CE-MRA and a longer TE with TOF-MRA.
The weighted κ values for the 2 MRA readers compared with each other for the outcome of aneurysm remnant class were as follows: 0.64, 0.57, 0.61, and 0.56 for 1.5T TOF, 1.5T CE, 3T TOF, and 3T CE, respectively. For the outcome of change since the previous angiogram, they were 0.30, 0.17, 0.23, and 0.23, respectively. Weighted κ values for the 2 catheter angiogram readers compared with each other were 0.64 for aneurysm remnant class and 0.58 for change since previous angiogram. An example demonstrating the issue of interobserver variability is given in Fig 4.
DSA (A), 3T TOF-MRA MIP (B), and 3T CE-MRA MIP (C). A coiled supraclinoid aneurysm remnant (white arrow, A; white circle, B and C) demonstrates the inherent variability in the application of outcome scales. Despite being well-depicted with all imaging techniques, there was disagreement in aneurysm class by the 2 DSA readers, by the 2 MRA readers, and between the adjudicated DSA result and the adjudicated MRA result. Digitally subtracted coils are seen in A (black arrows).
Discussion
Using clinical MRA protocols and rigorous adjudicated image interpretation conditions, we studied the accuracy of both CE-MRA and TOF-MRA at 1.5T and at 3T for the detection of coiled aneurysm remnants compared with a reference standard of catheter DSA. Findings of prospective trials of test performance of TOF and CE-MRA at field strengths of both 1.5T and 3T relative to DSA have not previously been well established in the literature. In the detection of any aneurysm remnant (classes 2, 3, or 4), sensitivities were good for all 4 MRA techniques.
However, sensitivity alone may not represent the most clinically relevant metric in evaluating MRA for treated aneurysm surveillance. Detection and characterization of relatively larger aneurysms is of critical importance in treatment decisions. Smaller remnants are typically not treated, whereas larger remnants may prompt additional therapies, either repeat coiling or surgical clipping.3,21 When the performance of MRA in detecting these larger and thus clinically more worrisome aneurysm remnants was evaluated, sensitivities diminished; this change is largely explained by MRA misclassification of class 3 aneurysm remnants as class 2. However, CE-MRA at both field strengths had greater sensitivity (ie, less underclassification) for these larger (class 3 or 4) aneurysm remnants than TOF-MRA at 1.5T. This diminution in sensitivity may be related to greater flow-related artifacts within larger aneurysm remnants with TOF-MRA compared with the luminal contrast-filling mechanism of aneurysm characterization with CE-MRA.
In any event, based on these findings, implementation of CE-MRA may be of clinical benefit over TOF for detecting clinically relevant remnants. TOF-MRA first identifies the location of coil masses better, however, and provides another view in case of a suboptimal CE-MRA contrast bolus and thereby adds value to the follow-up examination. Our preference would also be to perform TOF and CE-MRA at 3T, if a choice between field strengths existed. This preference for the 3T field strength is based on our demonstrated superior sensitivity of CE-MRA at both field strengths relative to TOF-MRA at 1.5T only, theoretic physical advantages (discussed further below), and previously demonstrated superiority of 3T MRA for uncoiled aneurysms relative to 1.5T.22
Specificities of the MRA techniques for detecting any aneurysm remnant were moderate but substantially improved for the detection of class 3 or 4 aneurysm remnants. Results using the other MRA outcome variable measured (ie, determining coiled aneurysm change since comparison DSA) gave substantially poorer sensitivities but improved specificities.
Several other investigators have studied the accuracy of various MRA techniques relative to DSA in the detection of aneurysm remnants or recurrences after endovascular coiling. In fact, reports have even suggested that MRA can be more accurate than DSA in some circumstances.13,14,23 A meta-analysis by Kwee and Kwee from 20076 included 16 studies of moderate methodologic quality and found pooled sensitivities of TOF and CE-MRA of 83.3% and 86.8%, respectively, and pooled specificities of 90.6% and 91.9%, respectively. The sensitivities of the 4 MRA techniques in our study were very similar to these pooled results, and the specificities of our techniques for class 3 or 4 aneurysm remnants were similar to slightly worse in our study compared with their pooled results. Our specificities for smaller aneurysm remnants were significantly worse, and this could be related to an expected greater interobserver variability in the evaluation of small or equivocal aneurysm remnants. Because of different approaches to analysis among published studies, it is often difficult to compare sensitivity for the larger more clinically relevant aneurysm remnants.
Since the Kwee and Kwee meta-analysis,6 there have been several other studies published on the topic of MRA for coiled intracranial aneurysms.7–19,24 In summary, some of these studies showed results similar to those in our current study, and some showed the superior performance of MRA relative to DSA compared with that in our current study, though some of these differences are very likely related to the differences in the aneurysm occlusion scales used. Conflicting conclusions regarding the performance of MRA relative to DSA and of the performance of various MRA techniques relative to each other certainly still exist, however.
Theoretic considerations may explain the variable performances of 1.5T and 3T MRA and TOF and CE-MRA. Because SNR scales approximately linearly with the main magnetic field (B0), SNR approximately doubles at 3T compared with 1.5T. Additionally, the “T1 dividend” at 3T, which exists because of greater T1 relaxation times of gray and white matter at 3T, makes it easier to suppress stationary brain tissue signal intensity relative to blood, thereby improving the depiction of blood vessels.25 The greater SNR at 3T can be translated into greater spatial resolution as well as contrast resolution, and this would be theoretically important in accurately depicting small coiled aneurysm remnants. Indeed, the image quality of uncoiled intracranial aneurysms has been demonstrated to be superior at 3T TOF-MRA relative to 1.5T.22 A final advantage of 3T MRA over 1.5T is the potential of better exploitation of parallel imaging, which can greatly decrease scanning times.
Notwithstanding the considerations listed above, an increased SNR at 3T also implies an increased artifact-to-noise ratio.26 Susceptibility changes in hertz are doubled at 3T relative to 1.5T, which is theoretically important when imaging small aneurysm remnants next to susceptibility effect−inducing coil masses.27 This could be mitigated by increasing the receiver bandwidth at 3T, because doubling the bandwidth at 3T restores chemical shift artifacts to the same amount as at 1.5T, though at a cost of a square root of 2 in SNR.28 However, because the T2* dephasing effects associated with susceptibility artifacts are per voxel, we could also use the increased SNR at 3T to achieve smaller voxel sizes and thereby mitigate increased susceptibility effects.29
TOF-MRA is easy to perform and does not require a gadolinium contrast agent injection. However, this technique has a loss of signal intensity within an aneurysm remnant related to turbulent flow and resultant intravoxel dephasing or to slow flow and resultant spin saturation. There are various ways of partially mitigating against this through tailoring pulse sequence and other examination parameters, such as minimizing the TE.2,23,30,31 Successful CE-MRA requires a robust and well-timed bolus gadolinium injection, but because its signal intensity depends on aneurysm filling with gadolinium, there may be less flow-related artifacts such as from saturation effects. Venous opacification, particularly within the cavernous sinus, and aneurysm wall enhancement, however, can both complicate interpretation of CE-MRA. Because of diminished susceptibility effects related to shorter TE values with CE-MRA than with TOF-MRA, there could be less obscuration of aneurysm remnants around platinum microcoils with CE-MRA. Both TOF and CE-MRA are T1-dependent techniques, and so methemoglobin in subacute thrombus within an aneurysm could falsely mimic flow.
There are several limitations to this study. First, it is difficult to measure accurately the technical outcomes of endovascular coil embolization.32,33 Objective measures such as coil packing attenuation or percentage of occlusion of the aneurysm volume are currently difficult to achieve, particularly in the clinical setting, so more subjective instruments, such as the visual and ordinal scale of Roy et al,20 must be used at present. The particular scale and the best number of response choices, however, are open to debate.
Cloft et al34 have shown that interobserver and intraobserver variability are inherent to the assessment scales of the completeness of coil embolization of intracranial aneurysms and that agreement improves with fewer response choices in the scales. Our MRA interobserver agreement was fair for the outcome variable of aneurysm class using 4 response options as was the agreement for the catheter angiogram interpretations in the article of Cloft et al when there were also 4 response options. Our MRA interobserver agreement was poor for the outcome variable of change since prior angiogram, which represents substantially worse agreement than that with the catheter angiogram interpretations in the article of Cloft et al for this outcome variable. The particularly poor interobserver agreement for change since prior angiogram in our current study may relate to the fact that more variables were introduced into our MRA analysis, particularly with regard to the differences in imaging technique (ie, MRA versus conventional angiography), such as in image planes and projections and the physical phenomena underlying a perceived aneurysm remnant, which add to variability in interpretation.
Ideal imaging equipment and MRA pulse sequence parameters are also a moving target, and image resolution and speed continue to improve with hardware and software improvements. For the purposes of this study and for the duration of the study, we “froze” the MRA parameters where they had been previously optimized for clinical use. Naturally, future studies with improved equipment may show improved performance of any MRA technique, and we would advocate continued investigations into the accuracy of MRA in the detection of coiled intracranial aneurysm remnants.
Conclusions
Larger more clinically important aneurysm remnants were better characterized with CE-MRA at both 1.5T and 3T compared with TOF-MRA at 1.5T. We would perform both TOF and CE-MRA in the follow-up of coiled intracranial aneurysms and at 3T if the choice exists.
Footnotes
Indicates Fellows’ Journal Club selection

-
This work was supported by the ASNR Neuroradiology Education and Research Foundation/Boston Scientific-Target Fellowship in Cerebrovascular Disease Research.
References
- Received August 4, 2009.
- Accepted after revision September 9, 2009.
- Copyright © American Society of Neuroradiology